Electrical conductivity
The total electrical conductivity of a material is the measure of its ability to allow the transport of an electric charge. There are two main types of charge carriers in materials – electrons and ions. Electrical conductivity arising from electron carriers is called electronic conductivity and electrical conductivity emanating from ionic charge carriers is termed ionic conductivity.
There are materials in which both types of carriers are involved in electrical conduction: mixed ionic-electronic conductors. The total electrical conductivity in such systems is the sum of its ionic and electronic conductivity.
In oxygen ion conductors, oxide ions are the predominant charge carriers. Oxygen ion conductors are used in many applications and have been studied extensively for solid oxide fuel cells (SOFC) devices. An interesting class of materials called Ruddlesden-Popper phases are being studied by researchers based at Imperial College by using neutron facilities at ISIS, and Oak Ridge in the United States, to understand the transport of oxygen in these materials.
Using neutrons
The capability of neutrons to probe species with low atomic numbers provides a unique ability to investigate oxygen transport in these materials. In contrast to X-rays, which interact with the electron cloud of the atom, neutrons interact with the atomic nucleus. The neutron scattering length of an oxygen atom is large enough to make them easily detectable by neutrons.
Researchers have previously pointed out the significance of neutron scattering in oxide ion conductors because it can be used to determine the crystallographic positions of the oxide ions, their atomic displacement parameters and their occupancies, which are otherwise invisible to X-rays. These properties are used later to understand the oxygen conduction mechanisms.
The group from Imperial collected high-quality neutron diffraction data at the POLARIS Beamline at ISIS and POWGEN at Oak Ridge, which gave information including the crystallographic location, occupancy, and the isotropic or anisotropic atomic displacement of the atoms. All these parameters are of particular interest in materials with high oxygen mobility.
Results
Three different compositions were studied:
- LaPr3Ni3O10-δ (L1P3N3)
- La2Pr2Ni3O10-δ (L2P2N3)
- La3PrNi3O10-δ (L3P1N3)
The researchers were able to visualise the possible transport pathways of the oxygen ions through these materials by measuring the occupancies of the oxygen ion sites, the distances between vacant oxygen sites and the atomic displacement parameters. They found that each of the three structures showed different pathways for the oxygen ions to move through the structure.
Another discovery that the team made was that, as well as the vacancies in the perovskite layers, they did observe some vacancies in the rock-salt layers of the L1P3N3 and L3P1N3 compositions. This is interesting, as earlier reports detailing structural studies of Ruddlesden-Popper phase materials mostly found vacancies in only the perovskite layers of these materials.
The group's work illustrates the importance of neutron scattering for understanding the fundamental defect chemistry of such materials. The insights gained by such studies could inform future tuning of the materials to have superior performance. The group will further complement their work with total scattering studies to probe the local structure around these sites.
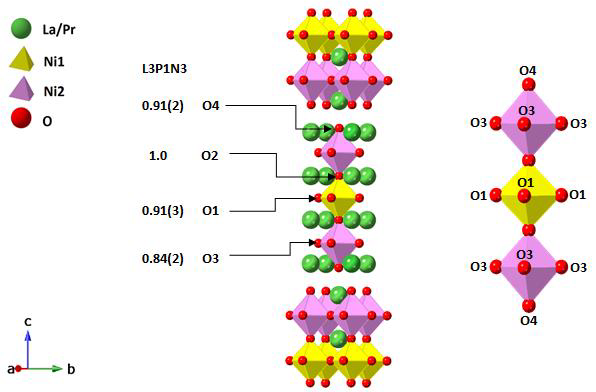
Figure 1. Ruddlesden-Popper unit cells of L3P1N3 tetragonal I4/mmm
structure. These structures are derived from Rietveld refinement of the neutron
diffraction data collected at 800˚C. The oxygen site occupancies are given
above along with magnified NiO6 octahedra bearing
labelled O sites.
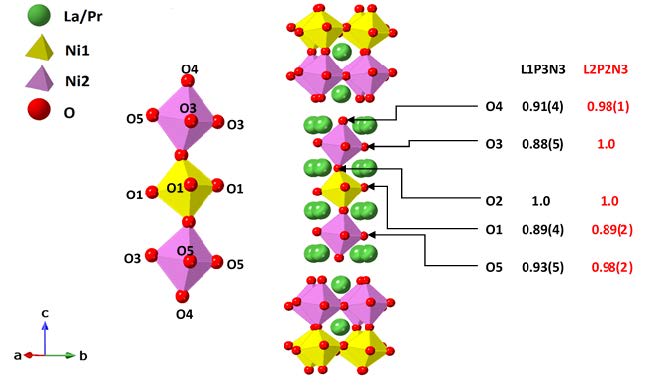
Figure 2. Ruddlesden-Popper unit cells for L1P3N3 and L2P2N3 orthorhombic Bmab structure. These structures are derived from Rietveld refinement of the neutron diffraction data collected at 800˚C for L1P3N3 composition and 600˚C for L2P2N3 composition. The oxygen site occupancies are given above along with magnified NiO6 octahedra bearing labelled O sites.
Further information
Read more science highlights from POLARIS here.