Thermoresponsive nanogels exhibit a drastic and discontinuous change of their physical properties with temperature. Professor Resmini's research group from Queen Mary University of London has a significant track record in the development of microgels and nanogels for applications as catalysts, sensors and drug delivery vehicles. In the past, they have successfully used neutron reflectivity to study the structural conformations of thermoresponsive nanogels and their interactions with lipid multi-bilayers on the molecular level.
The limited knowledge of the transport and adsorption dynamics of these nanoparticles at the interface, their structural conformation, and interaction mechanisms on the molecular level, limits further developments and potential applications of these materials.
The group has recently extended these studies to examine the behaviour of thermoresponsive N-n-propylacrylamide (NPAM) and N-isopropylacrylamide (NIPAM) based nanogels at an air/water interface. Although the monomers that make up these nanogels have similar chemical structures, the interfacial properties of NPAM based nanogels are significantly different from their NIPAM based counterparts.
NPAM exhibits higher hydrophobicity than NIPAM, and the amount of NPAM based nanogels adsorbed at an air/water interface, calculated from neutron reflectivity studies using the SURF beamline at ISIS, is higher than that of NIPAM based ones (Figure 1), suggesting that the overall hydrophobicity of the nanogels plays a significant role in their adsorption to the surface.
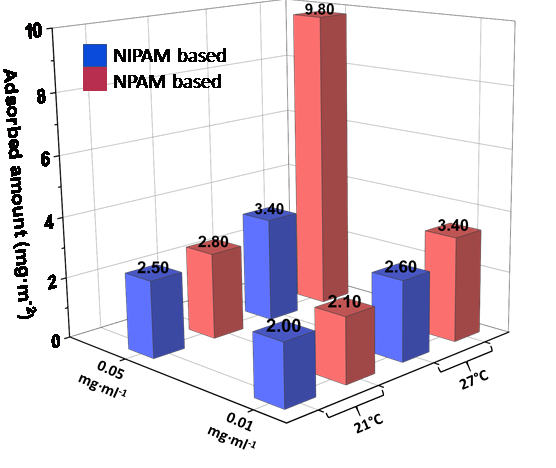
Figure 1. Adsorbed amount of NIPAM and NPAM based nanogels at the air/water interface as a function of temperature and concentration
The group also studied the properties of surface-charged nanogels at the air/water interface. By adding proline, the NAPM based nanogels become negatively charged. In this scenario, the researchers found a much lower percentage of nanogels at the air/water interface when compared with similar non-charged NIPAM based nanogels (Figure 2). This suggests the interaction of these charged nanogels with the water dominates over the interaction with the surface.
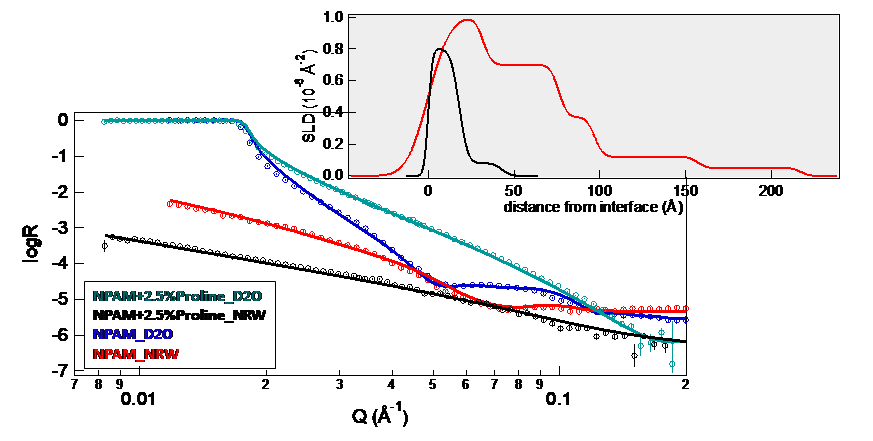
Figure 2. NR profiles and data fitting of NPAM and NPAM + proline based nanogels at the air/water interface. The insert is the SLD prolife in null reflectivity water (NRW) contrast.
The data suggest that small variations in a monomer's structure can result in different interfacial behaviour. The results contribute to the understanding of the adsorption of these stimuli responsive nanogels at interfaces, aiding the development of new applications for these smart materials.
A paper containing data mentioned above is in preparation and will come out soon.
