Green solvents
O. S. Hammond, D. T. Bowron and K. J. Edler, The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution, Angew. Chem., 2017, 56, 9782-9785.
O. S. Hammond, K. J. Edler, D. T. Bowron and L. Torrente-Murciano, Deep eutectic-solvothermal synthesis of nanostructured ceria, Nat. Commun., 2017, 8, 14150.
O. S. Hammond, D. T. Bowron and K. J. Edler, Liquid structure of the choline chloride-urea deep eutectic solvent (reline) from neutron diffraction and atomistic modelling, Green Chem., 2016, 18, 2736-2744.
Deep Eutectic Solvents (DES) are a sub-category of ionic liquids that only came to prominence at the turn of this century. These remarkable liquids are formed by the complexation of a hydrogen bond-capable salt with a neutral hydrogen bond donor species. In contrast to most room temperature ionic liquids, DES can be formulated as truly green designer-solvent media, for example the DES formed from a 1:2 molar mixture of choline chloride and urea, is a biodegradable, bactericidal and non-cytotoxic solvent. As hydrogen bonding is the fundamental driver of the properties of these liquids, the specialised capabilities of SANDALS and NIMROD make these instruments the perfect DES characterisation tools. This utility has been demonstrated in a recent series of studies performed on the choline chloride-urea system that have delivered a remarkable insight into the nanoscale structural organisation of the molecular components. The results of these experiments have additionally shown how the specific solvent properties can be tuned by the addition of water to the solvent mixture, and consequently how this can be used to deliver exquisite synthetic control of the nanoscale morphology of an industrially important catalyst, ceria.
Illustration of how the nanostructure of the Deep Eutectic Solvent, formed from a 1:2 molar mixture of choline chloride and urea, can be modified by the addition of water. Up to very high water content, the water molecules are found to be sequestered in localised domains around the cholinium cations in the bulk DES solvent matrix.
Multiscale structural studies of porous ices
C. Mitterdorfer, M. Bauer, T. G. A. Youngs, D. T. Bowron, C. R. Hill, H. J. Fraser, J. L. Finney, and T. Loerting, Small-angle neutron scattering study of micropore collapse in amorphous solid water, Phys. Chem. Chem. Phys., 2014, 16, 16013–16020.
C. R. Hill, C. Mitterdorfer, T. G. A. Youngs, D. T. Bowron, H. J. Fraser, and T. Loerting. Neutron Scattering Analysis of Water's Glass Transition and Micropore Collapse in Amorphous Solid Water, Phys. Rev. Lett., 2016, 116, 215501.
Porous Amorphous Solid Water (ASW) is the dominant phase of ice in the universe and as such can play a pivotal role in astro-physical and chemical processes in the earliest stages of planet building. An understanding of the phase and porosity of the ice is vital in correctly modelling these processes. The wide Q-range and faster run times on NIMROD have been used to simultaneously study the atomic and mesoscale structure of ASW upon heating. High Q data reveal the overall ice phase change (ASW to cubic), while low Q data highlight changes in pore morphology (cylindrical to lamellar) allowing an important insight into the nature of the glass transition in ASW.
Two dimensional nano-materials
C. Cabrillo, F. Barroso-Bujans, R. Fernandez-Perea, F. Fernandez-Alonso, D. T. Bowron, and F. Javier Bermejo. Absorbate-induced ordering and bilayer formation in propanol-graphite-oxide intercalates, Carbon, 2016, 100, 546–555.
Intercalation of graphene oxide, a precursor to graphene synthesis, can provide a route to prepare porous materials with tunable pore size. NIMROD was used to study in real-time the intercalation and vacuum de-intercalation of liquid 1-propanol within graphite oxide. Increased ordering upon intercalation and the formation of a stable nanostructure after heating under vacuum to 350K were observed in situ from simultaneous and independent measurements of the amount of un-intercalated 1-propanol (low Q), changes in the graphene oxide intercalated structure (intermediate Q) and measurement of 1-propanol intercalation loading (high Q).
V. Tudisca, F. Bruni, E. Scoppola, R. Angelini, B. Ruzicka, L. Zulian, A. K. Soper, and M. A. Ricci. Neutron diffraction study of aqueous Laponite suspensions at the NIMROD diffractometer, Phys. Rev. E, 2014, 90, 032301.
The multiscale structure of Laponite suspensions in arrested-gel (1.5 wt%) and glassy states (3 wt%) have been studied over the broad Q-range of the NIMROD instrument. The low Q structure matches that measured by other techniques (SAXS, DLS), and the intermediate Q-range for which NIMROD is optimised (0.2 < Q < 1 Å-1) reveals a clear signature of aging. The higher Q-range (Q > 1 Å-1) has been used to study the structure of water molecules within the layers closest to Laponite platelets surface. This shows orientational and translational order, which maps into the crystalline structure of Laponite.
Catalysis
T. G. A. Youngs, H. Manyar, D. T. Bowron, L. F. Gladden, and C. Hardacre, Probing chemistry and kinetics of reactions in heterogeneous catalysts, Chem. Sci., 2013, 4, 3484-3489.
Studying hydrogenation reactions in situ on NIMROD has shown that the instrument is capable of revealing spatial as well as kinetic information on the underlying processes taking place. Through analysis of changes within specific Q regimes over sequential short runs allowed a basic set of rate equations to be deduced for the hydrogenation of benzene over Pt/MCM-41. This has illustrated the utility of NIMROD to the catalysis industry, as well as pushed the boundaries of structural studies of confined liquids.
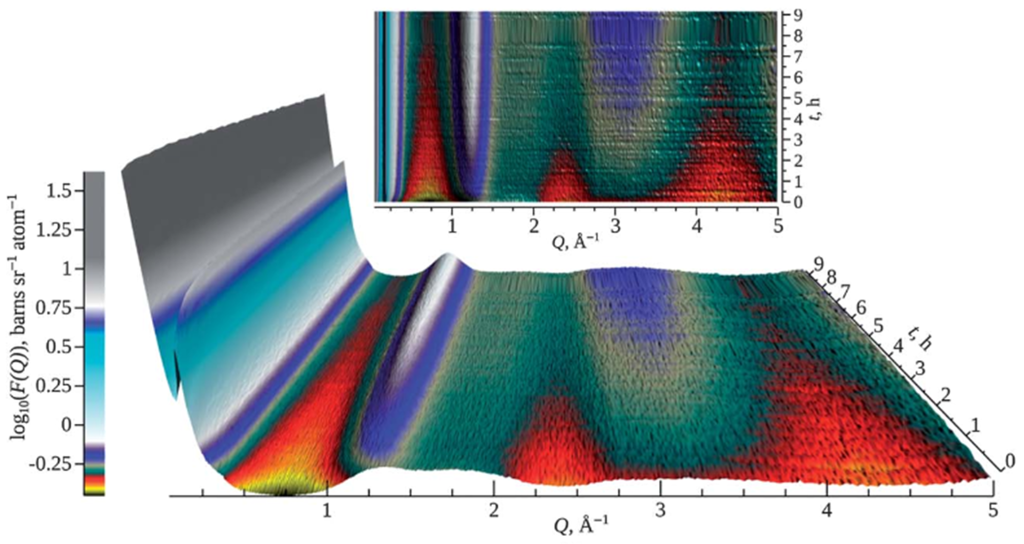
Time resolved NIMROD scattering curve for the hydrogenation of benzene.
Confined Fluids
K. L. Stefanopoulos, F. K. Katsaros, Th. A. Steriotis, A. A. Sapalidis, M. Thommes, D. T. Bowron, and T. G. A. Youngs, Anomalous Depletion of Pore-Confined Carbon Dioxide upon Cooling below the Bulk Triple Point: An In Situ Neutron Diffraction Study, Phys. Rev. Lett., 2016, 116, 025502.
K. L. Stefanopoulos, T. G. A. Youngs, R. Sakurovs, L. F. Ruppert, and Y. B. Melnichenko, Neutron Scattering Measurements of Carbon Dioxide Adsorption in Pores within the Marcellus Shale: Implications for Sequestration, Env. Sci. Tech., 2017, 51, 6515-6521.
NIMROD has revealed the somewhat surprising behaviour of liquid CO2 confined within porous substrates, providing direct new insight on the phase behaviour of nanoconfined fluids. Confined within the ordered mesoporous silica SBA-15, upon cooling below its triple point the CO2 escaped the porous matrix, rather than freeze or remain liquid. Furthermore, in a sample of the Marcellus shale it was shown that CO2 exhibits liquid-like properties within the porosity of the rock at sub-critical conditions, with important consequences for the feasibility of CO2 sequestration in such reservoirs.
Very large self-assembled structures
A. K. Soper and K. J. Edler. Coarse-grained empirical potential structure refinement: Application to a reverse aqueous micelle, Biochim. Biophys. Acta, 2017, 1861, 1652-1660.
Data from a reverse micelle system were analysed by means of a coarse-grained version of empirical potential structure refinement (CG-EPSR). Reasonable agreement was observed with the low Q data across six isotopic variants and two system concentrations. The resulting simulations show a large non-linear increase in micelle size with increasing water concentration. Importantly the study shows that extending EPSR to the longer length scales probed with NIMROD using such a coarse-grained simulation approach is feasible, and that extensions of the method could be applied to other systems such as polymers.
Our Science Highlight articles cover the diverse research that takes place at our facility. Read all our Science Highlight articles using NIMROD below:
