When you think of oil extraction, chances are the first image that comes to mind will be of an oil rig with a fountain of the black stuff bursting from deep within the Earth, driven out of the ground by billions of years of pent-up pressure, or maybe a huge mechanical pump syphoning oil from the deep. In reality, only about ten percent of the oil from a reservoir can be extracted in this way – during the phase known as primary recovery.
To extract more oil, secondary techniques are employed that involve injecting high-pressure water or gas into the well, displacing the oil and driving it to the surface. However this results in the recovery of only 20 to 40 per cent of the oil, which means as much as 60 per cent remains out of reach – locked away in cracks and fissures or within porous rock itself. Extracting this oil requires a third stage of extraction, known as Enhanced Oil Recovery (EOR).
There are several existing EOR techniques, which can involve pumping steam, or gases (such as carbon dioxide) into the system, but the new research has focused on a method known as surfactant flooding. This involves injecting a mixture of water and surfactants (the compounds used in detergents to remove dirt and oil) into the well – a process not to be confused with hydraulic fracturing, or fracking. The surfactants reduce the surface tension of oil, which makes it less clingy and more easily displaced by water – it also increases the ‘wettability’ of porous rocks (such as limestone), which allows the water to penetrate more easily. Industry has made multiple efforts to synthesise the ideal surfactant with the right combination of properties: it must interact in just the right way with oil and rock; be tolerant to salt (the water injected is usually a salty brine solution); and it must be able to withstand the much higher temperatures found deep underground.
Unfortunately, the surfactants most commonly used at the moment are fluorocarbon-based, which are costly, hazardous and environmentally damaging. The surfactant developed by the team from Swansea University is hydrocarbon-based, which they hope has the potential to be a ‘green’ replacement. To find out, the team, led by Dr Shirin Alexander and Professor Andrew R Barron, turned to the investigative powers of the ISIS Neutron and Muon Source at Rutherford Appleton Laboratory.
To test the new surfactant the team simulated the internal structure of a cross section of sandstone by etching a pattern onto a glass plate with a laser and then covering it with an un-etched plate. Using a technique called small-angle neutron scattering (SANS), which uses neutrons to explore the microstructures of liquids and solids, they then studied how well the surfactant solutions permeated the simulated sandstone and how effectively they displaced oil.
They tested a variety of solutions – a salty brine solution by itself, pure surfactant, and a mixture of brine and surfactant. They found that, when mixed with brine, the new surfactant improved oil recovery by 72 per cent – a huge improvement over the 45 per cent recovery of the other surfactants they tested. The result is all the more impressive because to achieve this level of recovery in the past, surfactants have needed to be combined with other materials, such as polymers.
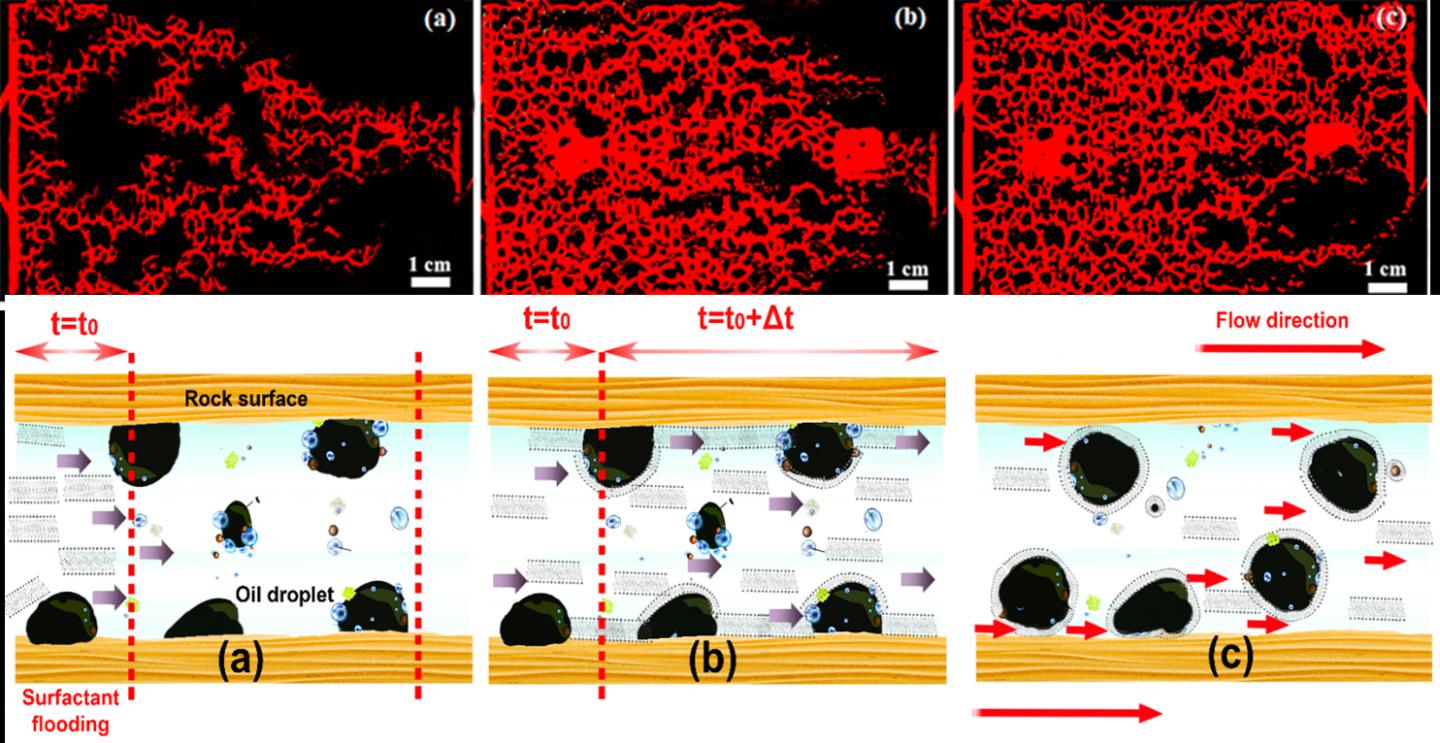
This shows how effectively oil was displaced through the micro-channels in the simulated sandstone by (a) pure brine, (b) pure surfactant, and (c) a surfactant-brine solution. Credit: Sajad Kiani, Swansea University.
Polymers are added to increase the viscosity of water – meaning it can push more oil of pores in the oil-bearing rock. Unfortunately, this increase in viscosity can make the water more difficult to reuse and the mixture of polymers and surfactants has to be disposed of instead. Since polymers do not easily decompose and surfactants themselves can be poisonous to wildlife, they can have potentially harmful and long-lasting environmental impacts.
Despite the increased societal desire to shift to renewable energies, the global demand for oil is not expected to decrease any time soon. The development of a surfactant that is based, not on harmful fluorocarbons, but on hydrocarbons, combined with the elimination of the need for additives, such as polymers, could be hugely significant for the oil industry and for the environment.
The full research paper is available here.
Article originally part of STFC's Fascination newsletter.
