In research funded by the Engineering and Physical Sciences Research Council, new molecular structure data collected at ISIS shows how mixing glycerol with water lowers the freezing point of water by preventing water molecules from forming into rigid ice networks. This new fundamental understanding of the role of glycerol will be helpful in a range of applications.
Group leader, Dr Lorna Dougan, from the University of Leeds said: “Knowledge generated in this area will improve our fundamental knowledge of cryopreservation which may lead to improved storage and recovery of tissue for fertility treatment, better storage of drugs in the pharmaceutical industry and transport of organs for surgery, and better storage of food in the agricultural industry.”
Cold-blooded animals such as lizards have little or no ability to regulate their own body temperature. When temperatures fall in winter, so does their body temperature, putting their tissues, cells and biological activity at risk of irreparable damage from ice crystals.
Faced with this situation, some animals choose to avoid the cold by migrating to warmer climates or hibernating, whilst others such as lizards have developed adaptive techniques to avoid becoming an ice statue.
To prevent lethal ice crystals forming in and between cells in their body, lizards use chemical compounds such as glycerol to reduce the freezing temperature of water. Glycerol is a common cellular component in many cold-blooded organisms. It acts as a cryoprotectant compound, protecting cells and tissues during prolonged exposure to subzero temperatures by effectively pausing cell activity until temperatures rise again and normal cell activity can safely resume.
The method of preserving cells or whole tissues by cooling to sub-zero temperatures (known as cryopreservation) is used in many industries, medical protocols and everyday items ranging from car antifreeze to reducing the amount of ice in your ice-cream.
Whilst the natural process has been replicated in laboratories for more than 60 years, understanding how the molecular mechanisms allow glycerol to provide this cryo-protection is still limited.
Researchers from the Dougan Research group at Leeds University have used neutron diffraction data from ISIS alongside computer modelling to look in detail at glycerol’s molecular mechanisms, in particular how glycerol-hydrogen bonds form.
Dr Dougan added: “Neutron diffraction is ideal for the structural study of liquid glycerol. Over the past decade, significant advances have been made in the methods of neutron diffraction and in the development of more powerful computational tools. Our study provides the first detailed experimental, structural insight and allows a number of hypotheses to be tested for the first time.”
The ISIS data revealed that the presence of only small quantities of glycerol in water had the same impact on the water structure as increasing the pressure – reducing the freezing temperature of water and preventing the formation of ice.
The team has now begun a new collaboration with Dr Giovambattista from City University of New York, an experienced researcher in the fields of supercooled and glassy systems. They are also engaged with researchers who use cryopreservation for fertility treatment in reproductive medicine and in the storage of transplant organs. Whilst cryopreservation protocols to freeze-store these cells and complex tissues are already in use, the methods used will greatly benefit from further investigation and optimisation.
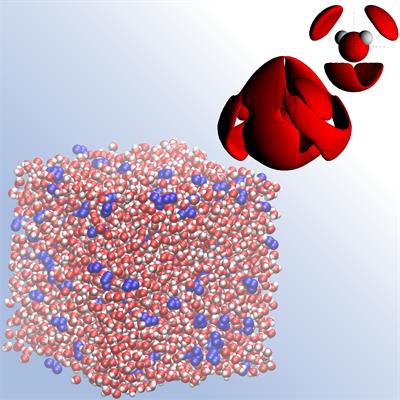
A snapshot of molecular mixing in a dilute glycerol – water solution, where glycerol molecules are depicted in blue and water molecules in red. The zoom in (on the upper RHS) shows the spatial density of water molecules around a central water molecule. We can use the snapshot and the water spatial density function to learn more about how glycerol water structure.
View full-size image
Dr Lorna Dougan
Research date: August 2011
Further Information
This research was funded by the Engineering and Physical Science Research Council and is one of the research stories featured in this month's healthcare themed STFC newsletter 'Fascination', available at the end of February http://www.stfc.ac.uk/fascination
This research has been published in the Journal of Physical Chemistry B.
The Engineering and Physical Science Research Council are supporting this research to enable the full team PhD student James Towey, ISIS scientist Professor Alan Soper and group leader Dr Lorna Dougan, School of Physics, University of Leeds) to conduct this research.
For more information, please see the Dougan Research Group website
http://www.mnp.leeds.ac.uk/ldougan/research.html
