Diffusing within the membrane “track”, it passes on a “baton” of electrons to its drug metabolising team-mates, the cytochrome p450s, by stretching out its protein “arm” changing its conformation.
Inside every cell in our body lies a workforce of little helpers that keep us ticking – from helping us to breathe to digesting the food we eat. Theses clever little molecules are called enzymes and some of them work long shifts in the liver to metabolise drugs.
The CPR enzyme, which contains two flavins, is involved in this drug metabolising system. In the liver there are a whole lot of cytochrome P450s – their mission is to take an oxygen atom and add it to drugs as a step towards inactivating them, but in order to do this they need electrons. This is where the CPR enzyme “runner” comes in. Acting as the middle man, it grasps the electron “baton” from a co-enzyme onto one of its flavins, passes it to a second flavin in its other “arm” domain and then diffuses around in the membrane “track” encountering different cytochrome P450’s and passes the electrons on to them.
A group of scientists from the University of Leicester have used small angle neutron scattering on the LOQ instrument at ISIS to uncover how this CPR enzyme exists in two forms, a compact and a more extended structure, in order for it to fulfil this relay function.
Dr Gordon Roberts (Department of Biochemistry, University of Leicester) explains “This enzyme has three folded domains. In the compact structure seen in the crystal structure these domains are suitably positioned for transferring electrons internally from a coenzyme to a flavin on one domain and on to the second flavin in another. However it has been clear for a while now that there has to be a conformational change in CPR in order for it pass electrons from the flavins to the P450s.”
"Crucially, in the crystal, the flavin “arm” was tucked away in the middle of the protein. This raised the question; how could the P450 get to it? “There had to be a change in the arrangement of the domains of the enzyme for the flavins to be accessible to the P450” explains Gordon, “So this is what we set out to discover.”
Initially they used small angle X-ray scattering which indicated the existence of an equilibrium between a compact and more extended structure, but they realised that the X-rays were interfering by producing photo-electrons which reduced the flavins, which normally only get reduced by the co-enzyme.
Neutrons accelerate research into the dynamic domains of an enzyme involved in drug metabolism.
“That’s where ISIS came in” explains Gordon “With neutron scattering experiments there isn’t that problem of reduction – and in fact the results of the neutron experiments were more interesting than what we first expected.”
The expectation was simply to find a mix of compact and extended forms but the neutron experiments revealed that the domain movements were closely coupled to the enzyme's catalytic cycle. The oxidised enzyme that hasn’t yet received electrons was almost 85% in the compact state. The transfer of electrons from the co-enzyme to the flavins directly triggered domain movement to produce the extended conformation.
Understanding the enzymes involved in the drug metabolising system is vital; pharmaceutical companies are interested to know about this system as it influences how drugs are handled in the body. They want to know not just whether a drug binds to its target, but crucially – if it will get to its target and therefore, how is it metabolised?
One way in which the team want to further this research is to look at the CPR as it interacts with the P450 in the membrane. Gordon explains “It’s much more difficult to look at enzymes when they’re embedded in the membrane. However we are planning to do a joint project with the Institut Laue-Langevin using neutron reflectometry to look at the behaviour of these enzymes in the membrane.”
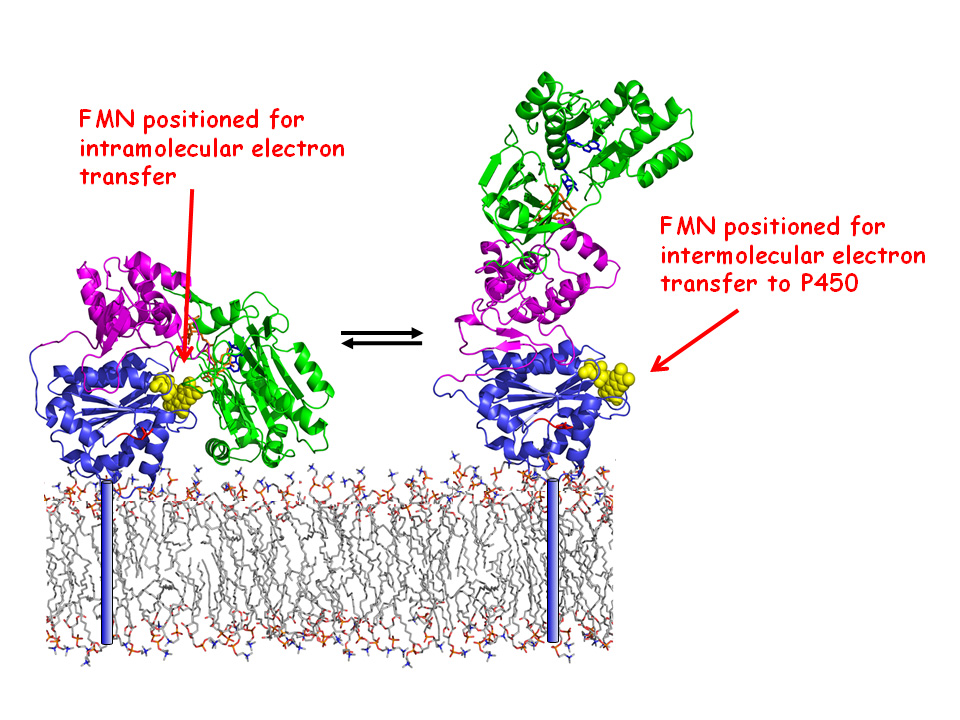
The team want to look at how the protein behaves in the membrane.
The CPR enzyme is not just involved in the drug metabolising system; it’s also a key player in steroid metabolism. “There are fortunately rare but extremely unpleasant syndromes where steroid metabolism is disordered” explains Gordon “A number of these syndromes can be traced back to mutations in the CPR. So the more we can learn about this enzymes function the more we can understand how these mutations can affect it."
Felice Laake
Gordon Roberts et al.
Research date: July 2013
Further Information
This research has been published in Structure.
Huang et al., Redox-Linked Domain Movements in the Catalytic Cycle of Cytochrome P450 Reductase, Structure, (2013).