By combining results from neutron diffraction, molecular dynamics simulations and quantum chemical calculations, a team of scientists have been able to characterise the hydrogen bond lengths in an ionic liquid. The group, a collaboration between universities in Germany and Australia and ISIS, found that, despite their charge repulsion, ions of the same charge formed stronger hydrogen bonds between them than ions that were oppositely charged.
A hydrogen bond is not a 'usual' chemical bond, but is an attractive force between a partially positive-charged hydrogen and a partially negative-charged atom, for example oxygen. Hydrogen bonds are responsible for keeping water molecules together, and give ice its open structure.
Unlike conventional molecular liquids, ionic liquids are made up entirely of ions and, intuitively, one might expect that the charge interactions between the ions dictate their properties. However, when taking into account hydrogen bonds and other weak interaction forces, the system becomes much more delicately balanced.
In ionic liquids where an O-H bond is present (hydroxy-functionalised), the hydrogen bonds make things much more complex, as the hydrogen bonding has a double-faced nature. This occurs when two distinct types of hydrogen bonding coexist: hydrogen bonds between the cations and anions (c–a) and those between like‐charged ions (c–c). It would be expected that the repulsive Coulomb force between the charges would cause the overall attraction between the like-charged ions to be weaker.
To study the ionic liquid, the group used neutron diffraction on the SANDALS instrument at ISIS, as it can be used to determine the structure of a liquid at very high resolution. By also substituting hydrogen for its heavier version, deuterium, the technique becomes very sensitive to the positions of hydrogen atoms. This enables the researchers to assess more directly the hydrogen atoms that are participating in each hydrogen bond. The deuterium lab based at ISIS carried out the substitution.
This study focussed on 1‐(4‐hydroxybutyl)pyridinium bis(trifluoromethylsulfonyl)imide [HOC4Py][NTf2], measuring its structure using neutron diffraction with isotopic substitution, as described above, with the aim to characterise the length of the hydrogen bonds between the cations and anions (c–a) and those between like‐charged ions (c–c).
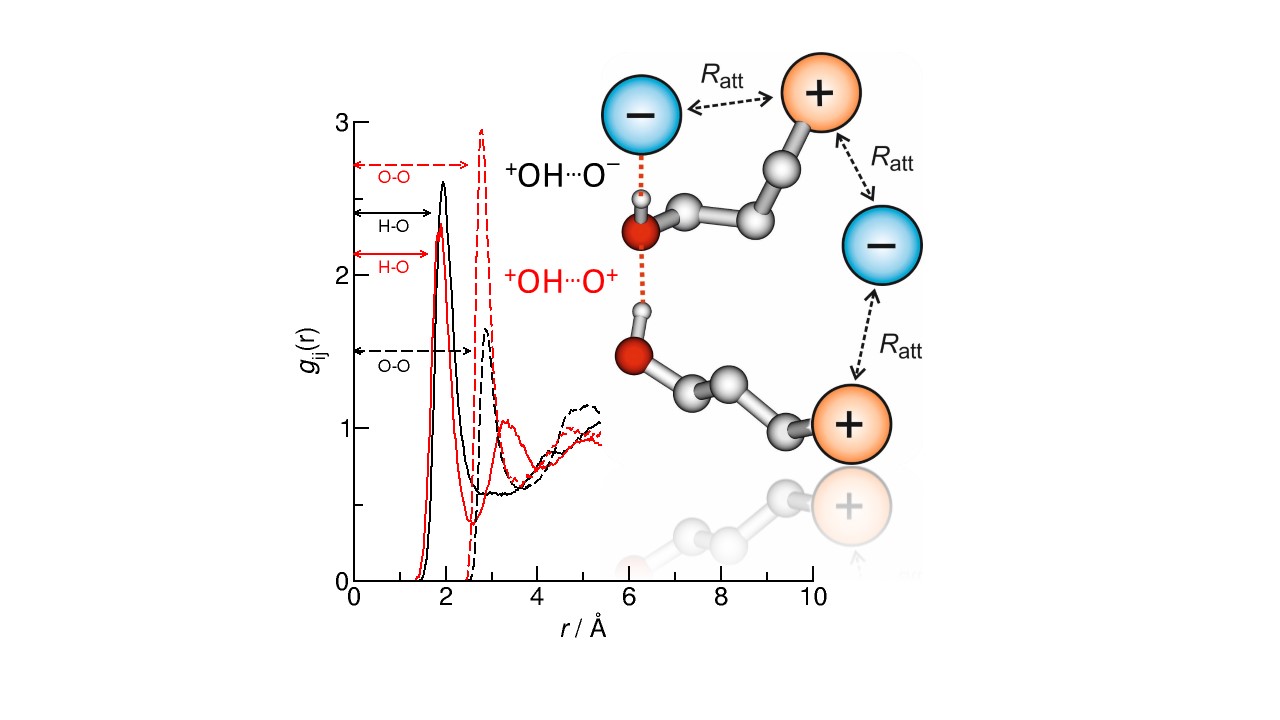
The group used molecular dynamics simulations to enable them to interpret the results of the neutron diffraction experiments. They found that the (c–a) hydrogen‐bond length in the ionic liquid is longer than found in water and ethanol, indicating weaker bonds despite the attractive force between the charges. In contrast, the (c–c) hydrogen‐bond lengths in the ionic liquid were shorter than found in water, indicating stronger bonds. This suggests that the O-H groups of the cations can form cooperative hydrogen bonds with each other nearly as well as in molecular liquids.
“ISIS is the prefect neutron source for showing hydrogen bonding between opposite- and like-charged ions in ionic liquids at the same time," explains Ralf Ludwig, Professor of physical and theoretical chemistry at the University of Rostock, Germany. The collaboration with his colleague Rob Atkin from the University of Western Australia provides the first hydrogen bond length between ions of like charge, and shows that they are surprisingly stronger than those in typical ion-pairs are.

Left to right: T. Niemann (Germany, Rostock), S. Gärtner (ISIS), P. Stange (Germany, Rostock)
Further information
Full paper: https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201904712
Other science highlights from SANDALS.
