Scientists from the University of St Andrews, AWE and ISIS have been able to determine the mechanism behind the transitions involved in the cathode of a thermal battery by studying the different phases formed and their structures during discharge. Understanding the structures of the materials involved has previously been challenging, due to the high temperatures at which these batteries operate.
Thermal batteries are used for storing electricity for times when demand requires a high power to be drawn out over a long period, such as in aircraft emergency power supplies. They are made up of a cathode, separator, a lithium-containing molten salt electrolyte and an anode. The electrolyte is solid at room temperature, which helps prevent the battery from slowly discharging before use. CoS2 is a commonly used cathode due to its high electronic conductivity and high thermal stability, and optimising its discharging will improve the performance of the battery as a whole.
Although the electrochemical properties of CoS2 and other similar cathode materials have been studied in detail before, the high temperatures and inert atmosphere needed for battery operation have prevented operando studies of the structures present in the cathode during discharge. A new sample mounting assembly on the Polaris beamline, designed and built by the St Andrews team, has allowed for the first time a structural study of thermal batteries at the higher temperatures needed for operation.
When in the presence of heavier transition metals, lithium atoms are much more visible to neutrons than with other scattering techniques. Therefore, by using neutron diffraction to study the structure of the CoS2 in a thermal battery environment, the group were also able to monitor whether lithium is involved in any of the intermediate stages of discharge.
Their experiment took the battery to 520°C and then discharged it from 1.8 V down to 1.0 V. They found that four different structures are formed as the CoS2 cathode discharges. By analysing their neutron diffraction data, the group could both identify and quantify the phases and their structures for the first time. They were able to track the amount of each phase present at each voltage and, from this, propose a mechanism for the discharge.
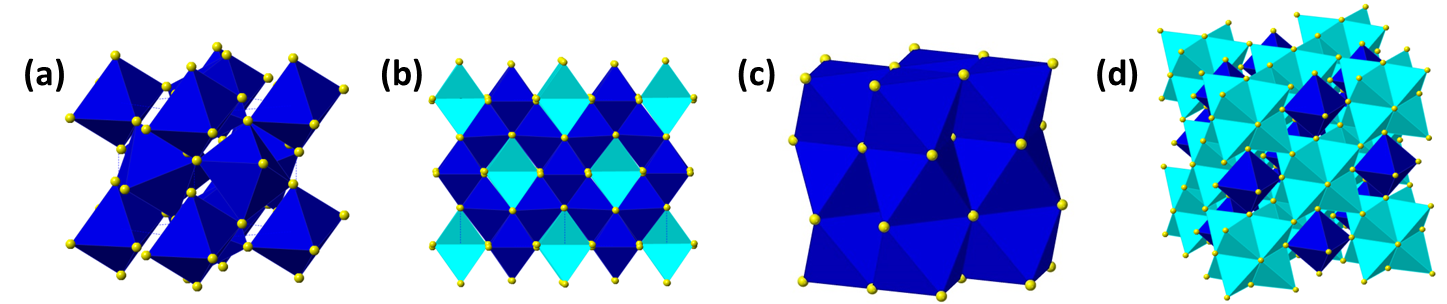
Crystal structures for different cobalt sulfides. (a) CoS2 with the pyrite structure, (b) Co3S4 with the spinel structure, (c) CoS with the NiAs structure and (d) Co9S8. Yellow atoms are sulfur, dark blue atoms are cobalt in octahedral sites and light blue is cobalt in tetrahedral sites.
Surprisingly, their results did not support a previously proposed mechanism, which included a Co3S4 intermediate phase. No Co3S4 was observed during the experiment but, instead, they saw evidence of CoS formation, suggesting that this phase is part of the discharge process. This result was particularly interesting, when considering earlier work by the St Andrews team looking at a thermal battery that used a NiS2 cathode. Despite Co and Ni being next to each other in the periodic table, it had been thought by others that, when discharging thermal batteries using CoS2 and NiS2 cathodes, different structures formed. By carrying out operando studies on Polaris, the team found that the first part of the battery discharge is the same for NiS2 and CoS2, as the same structure types were observed whilst discharging. This indicated that there were more similarities than originally thought.
The use of operando experiments on Polaris has been crucial in allowing the St Andrews team to understand these batteries in more detail and the team believes that these are the highest temperature operando studies that have been carried out to date.
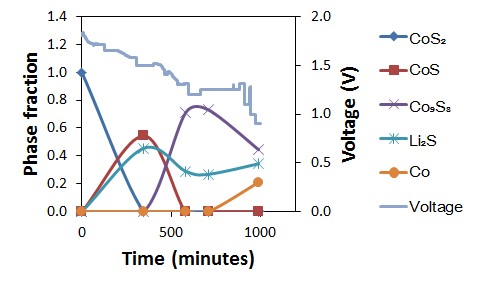
Phase fractions (as weight percent) extracted from multiphase Rietveld refinement showing evolution of phase fraction and voltage with time
Further information
http://jes.ecsdl.org/content/166/12/A2660.full.pdf+html (Open Access)
https://onlinelibrary.wiley.com/doi/full/10.1002/celc.201700095
https://www.frontiersin.org/articles/10.3389/fenrg.2018.00121/full
Other Science Highlights from Polaris.
Ministry of Defence © Crown Copyright 2019/AWE
