 Hydrogels are well-established as excellent candidates for biomedical applications, including wound treatment, tissue engineering and controlled drug release. While synthetic- and bio-polymers are used extensively as building blocks in hydrogels, the potential of folded and functional protein-based hydrogels is just beginning to emerge. This study demonstrates that single protein stabilisation, through ligand binding, can translate to the mechanical properties of the whole cross-linked protein network.
Hydrogels are well-established as excellent candidates for biomedical applications, including wound treatment, tissue engineering and controlled drug release. While synthetic- and bio-polymers are used extensively as building blocks in hydrogels, the potential of folded and functional protein-based hydrogels is just beginning to emerge. This study demonstrates that single protein stabilisation, through ligand binding, can translate to the mechanical properties of the whole cross-linked protein network.
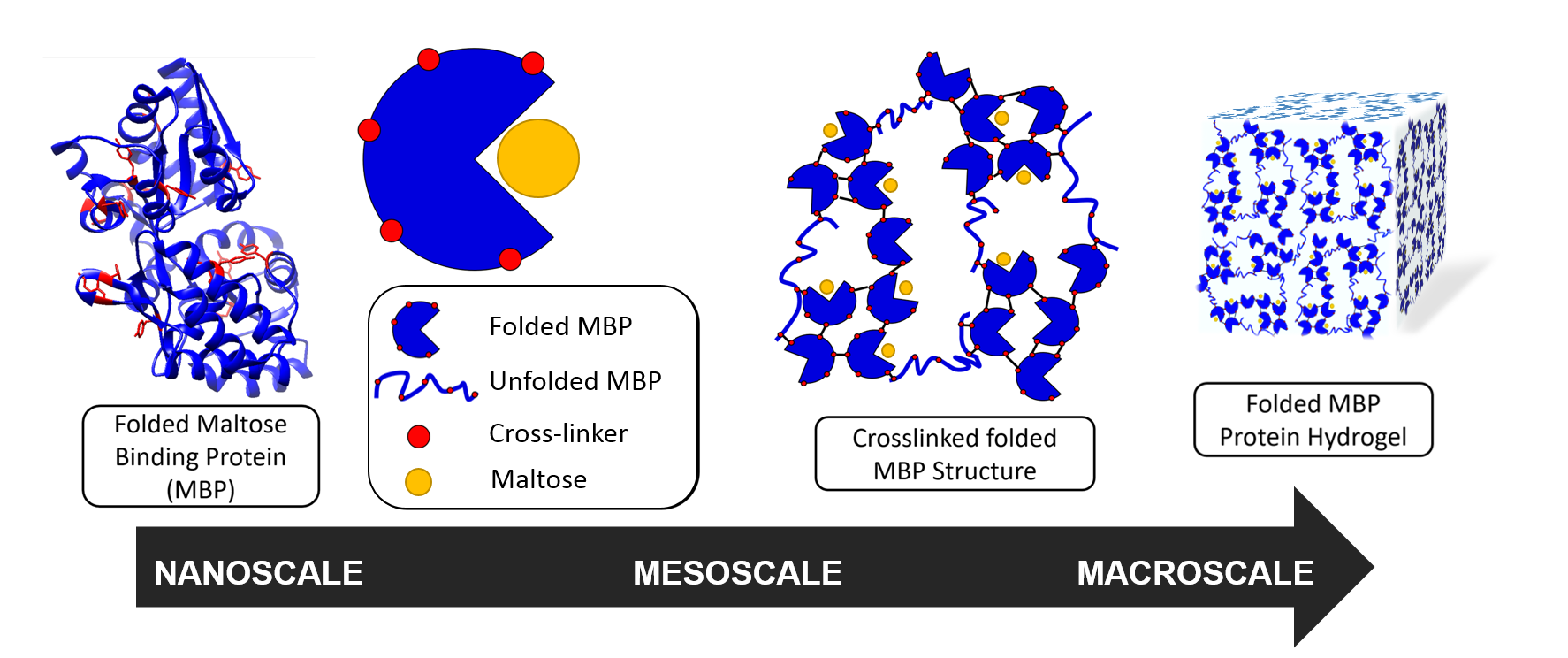
The diverse biological functionality of protein 'bionanomachines' and their inherent structure-function relationship offers huge potential for smart and responsive biomaterials. A major challenge is understanding how the properties of the biological building block, such as the folded protein, translates to the macroscopic properties of the protein network. In biological systems, this structural and mechanical hierarchy is crucial in providing diverse responsive functionality from the assembly of simple structural motifs.
Professor Lorna Dougan, University of Leeds, who leads the project said “Harnessing an understanding of the hierarchical biomechanics of folded proteins to create a programmable hydrogel would create transformative technology."
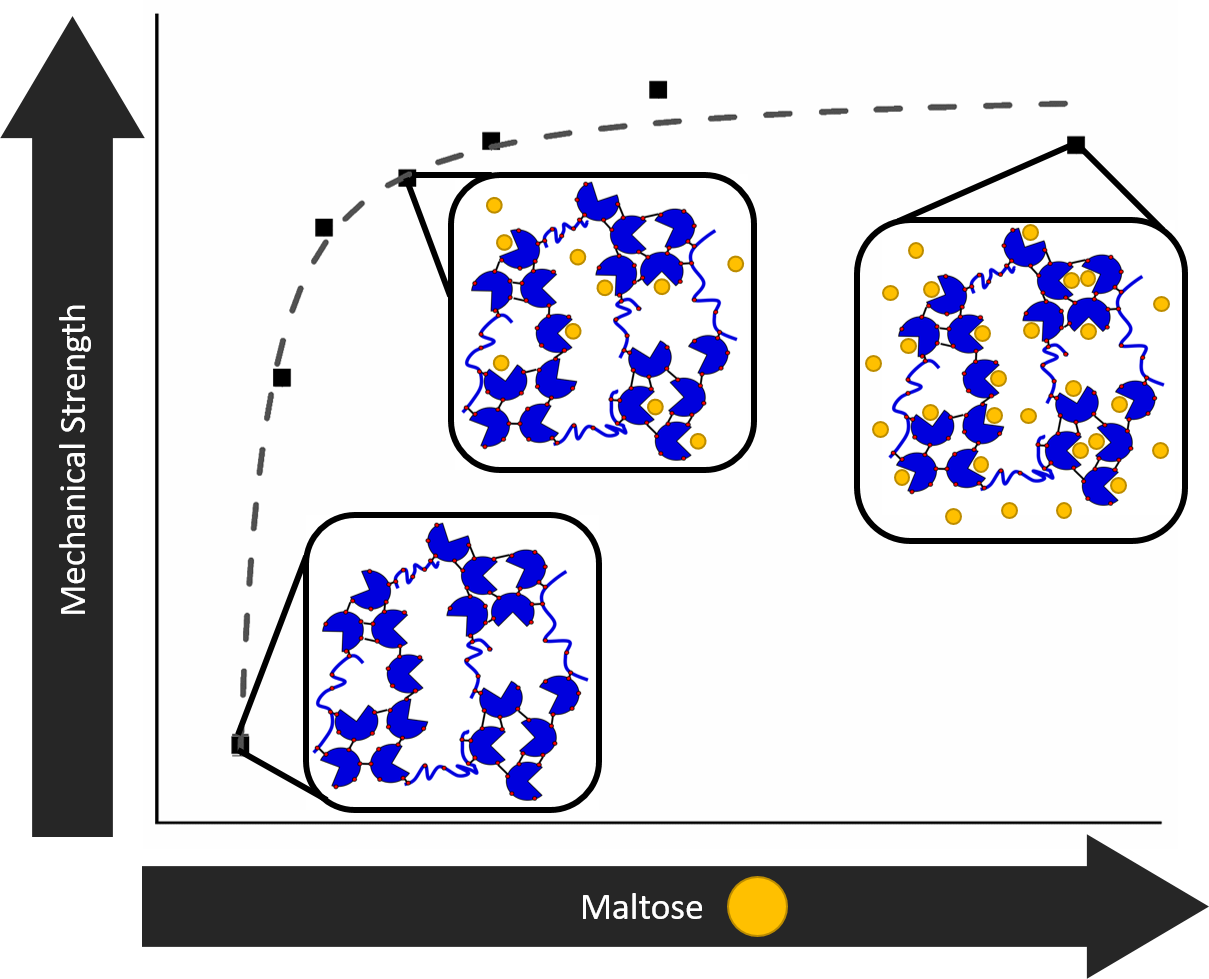
To be able to do this, it is important to understand whether modulation of the mechanical properties at the nanoscale has any impact at the macroscale. Her research team, including White Rose network funded PhD student Matt Hughes who spent his 2019 summer placement at ISIS, used maltose binding protein (MBP) as a model to investigate the relationship between building block stability and the macroscopic properties of a cross-linked MBP hydrogel.
When bound to maltose, the mechanical and thermal stability of the protein molecules can be increased. The group found that they were able to translate this stability of the protein building block into the cross-linked folded protein hydrogel, which showed increased mechanical strength.
Using small angle neutron scattering on LOQ and ZOOM, and small-angle X-ray scattering in the ISIS Materials Characterisation Laboratory, the researchers were able to see that the differences in the mechanical properties of the gel were not due to a mesoscopic structural change in the protein network. Instead they result from the increased proportion of more stable maltose 'occupied' MBP molecules.
As well as finding this increase in strength, the group were able to see other effects of maltose binding, and determine the structure of the hydrogel. The hydrogels contained fractal-like clusters of cross-linked folded MBP proteins, which are linked together by strands of unfolded protein, with an inter-cluster distance of approximately 1000 Å.
This ability to control the stability of the protein building blocks offers a route to controlling the mechanical and dynamical behaviour of a network of proteins, and therefore smart and responsive biomaterials with many applications.
Further information
The full paper can be found at DOI: 10.1039/C9SM02484K.
The project is part of a wider group effort by Professor Lorna Dougan at the University of Leeds (https://dougan.leeds.ac.uk/) to exploit multiscale mechanics of folded proteins in the design of new biomaterials, and is funded by an EPSRC Fellowship (EP/P02288X/1).
