 Proteins are crucial for a range of biological functions, with their structure and shape determining what role they are suited for. In some cases they are present on their own and in others, such as in structural proteins like collagen, they form networks. Understanding how the behaviour of individual protein molecules effects the whole network is crucial to both understanding biological functions and in developing new biomaterials.
Proteins are crucial for a range of biological functions, with their structure and shape determining what role they are suited for. In some cases they are present on their own and in others, such as in structural proteins like collagen, they form networks. Understanding how the behaviour of individual protein molecules effects the whole network is crucial to both understanding biological functions and in developing new biomaterials.
Researchers from the University of Leeds came to ISIS to study the process of protein unfolding and how this impacts the structure and mechanical properties of the wider network. Their work, published in ACS Nano, studied the protein bovine serum albumin (BSA) formed into a chemically cross-linked hydrogel. Individual BSA molecules are held together in the folded state by multiple internal disulphide bonds that act as nano-staples.
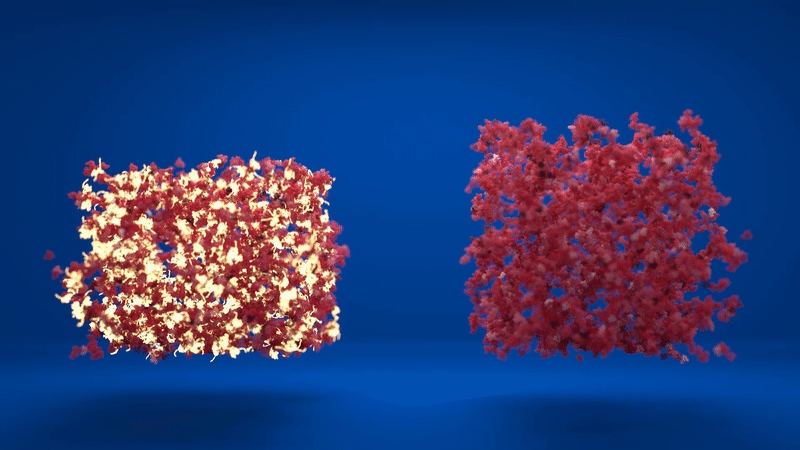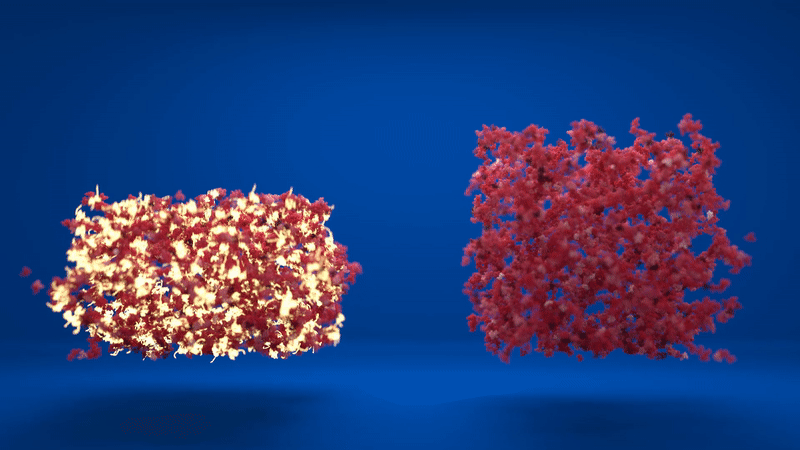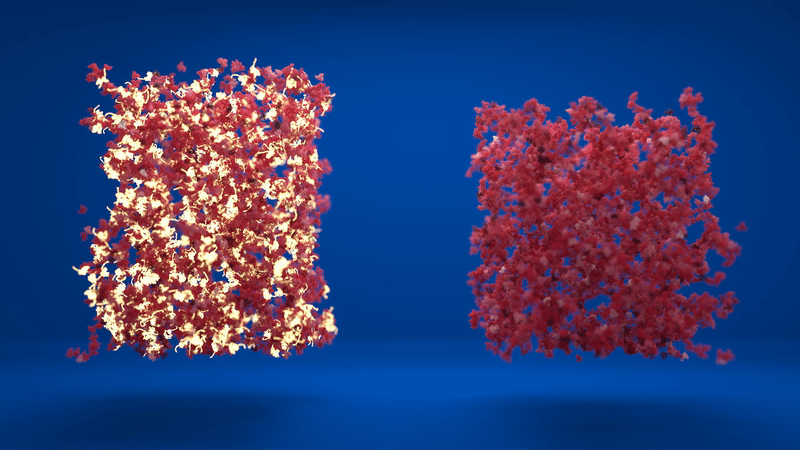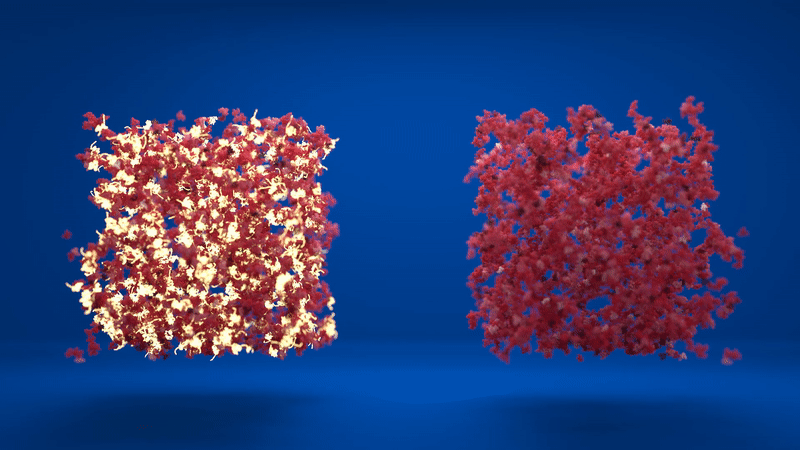
Above: Animation comparing the flexibility of two hydrogels which contain different amounts of folded and unfolded protein. Credit: Lorna Dougan, created by Phospho.
The researchers were able to chemically break the nano-staples, allowing the protein to unfold when it is connected in a network and experiences force. This enabled them to use the same protein building block to create biomaterials with distinct architecture and mechanical properties.
Using small angle neutron scattering (SANS) on SANS2d, and small angle X-ray scattering in the materials characterisation lab at ISIS, they were able to study the structure of the protein system across a range of length scales. From the data, they were immediately able to see that there was a significant change to the network's structure when the proteins were able to unfold during the formation of the hydrogel.
The hydrogel is made up of clusters of proteins, with loose proteins in between them. When the proteins were stapled together, the structure was more homogenous, with lots of folded proteins in the inter-cluster region. However, when the proteins were able to unfold, the clusters formed were much denser and the inter-cluster region between them contained unfolded protein.
The group also tested the mechanics of the gels, expecting the gel to get softer when the proteins could unfold. “Actually we saw the opposite!" explains lead author Matt Hughes; “the unfolded proteins form lots of connections between clusters, shifting the focus from the clusters themselves to what is happening in the regions between them."
Using their new modelling platform BioNet, developed by Dr Benjamin Hanson, the team looked to see what was happening at the molecular level. They found that it was the process of protein unfolding that made the difference. The change to the network structure was only seen when the unfolding occurred during gelation.
“The next stage would be to see if it is possible to tune the amount of unfolding and how this impacts the system," says Matt. “This could lead to the ability to engineer a material with specific architectural and mechanical properties."
“This study is a demonstration of the value that using SANS can bring to the folded protein hydrogel community who study these systems," adds Professor Lorna Dougan, group leader; “we have been able to use the technique to provide new structural insights, and this will prompt future studies in this field to look at the structure across the length scales and even expand to studies of the kinetics of the network formation. Our next goal is to exploit the wonderful biological functionality of the bionanomachine (the proteins) in the networks. "
Further information
The full paper can be found online at DOI: 10.1021/acsnano.1c00353
The project was supported by a grant from the Engineering and Physical Sciences Research Council (EPSRC) (EP/P02288X/1) to L. Dougan.
