Our skin acts as a barrier between the inside of our body and the external environment. The outer later, the stratum corneum, is crucial to this function. It is made up of lipid bilayers arranged in an ordered matrix. Disruption to this matrix is a key contributor to skin diseases with an impaired barrier, and so understanding how this happens is key to learning how to treat them.
In this study, published in Biochimica et Biophysica Acta (BBA) – Biomembranes, the researchers used neutron diffraction on the Larmor instrument to study a model skin membrane with almost atomic resolution.
Usually at ISIS, membranes are studied using reflectometry, where you can measure a single film in contact with water. In this experiment, the sample was supported on a silicon substrate and measured in a saturated water atmosphere. The sample itself was a spray coated film, hundreds of bilayers thick.
These conditions allow a precise measurement of the arrangement of the molecules in the system without the need for complex modelling. Analysing the data from a series of samples with different levels of hydrogen and deuterium, the researchers could probe the structure of the membrane.
By measuring multiple samples in this way, the researchers were able to find that the composition of the lipids affected significant the structure and therefore the behaviour of the lipid matrix. By changing the lipid headgroup, they saw changes to the dispersion of particular lipids within the membrane. This change in dispersion could be a factor in certain types of skin diseases.
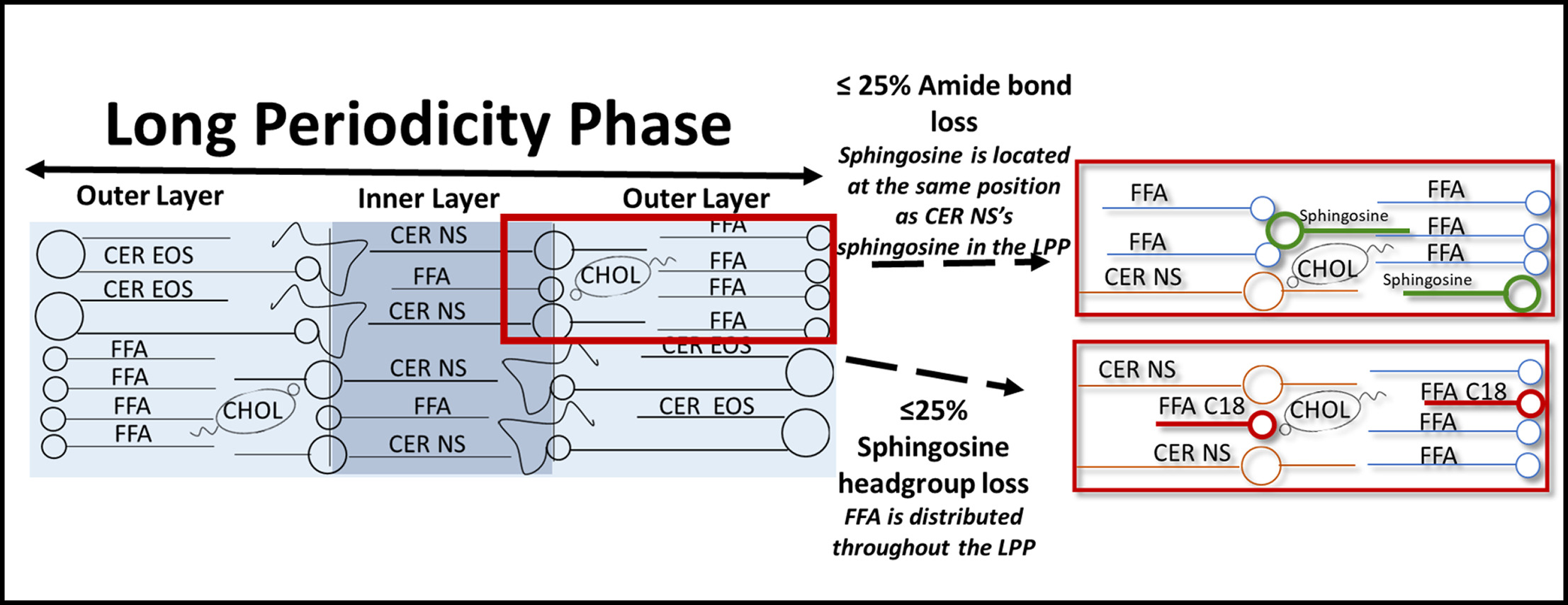
Schematic diagram of the lipid arrangement of the LPP both unmodified (left) and when under ≤25% Amide bond loss, with the substitution of sphingosine (green) and FFA C24 (blue) with CER NS (top right). Or ≤25% Sphingosine headgroup loss, with the substitution of FFA C18 (red) and FFA C24 (blue) with CER NS (bottom right).
Further information
The full paper can be found at DOI: 10.1016/j.bbamem.2022.183886
