Non-ionic surfactants are commonly added to pesticide formulations to enhance the quantity of pesticide that is taken up by a plant. The authors of this study have previously confirmed that pesticide molecules insert into the surface of the non-ionic surfactant micellar shell, due their amphiphilic nature, causing the micelle to change shape and become more hydrophobic. This study has indicated that these preloaded pesticide micelles are capable of interacting with the waxy surface of plants, dissolving the leaf waxes into the micellar core and triggering the release of pesticide molecules.

A small angle neutron scattering instrument (SANS2D) was used to examine how the structure of the non-ionic surfactant micelles changed when exposed to solubilised pesticide and waxes. SANS2D is not capable of locating the individual atoms, but rather provides a detailed overall structure of the micellar shell and core.
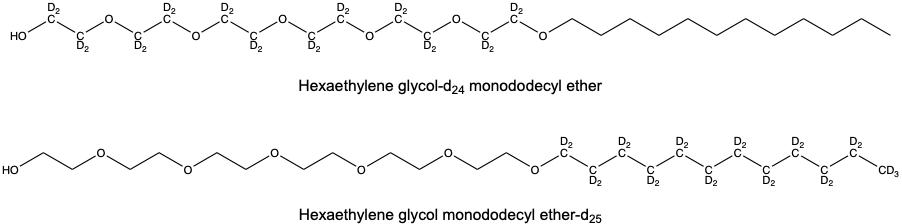
The Deuteration Facility supported this study by providing a chain deuterated and head deuterated surfactant called hexaethylene glycol monododecyl ether. These two deuterated surfactants enabled the authors to tune the scattering length density of the sample, in order to highlight different structural regions of the micelles when solubilised pesticide and wax was added. This process is commonly referred to as contrast matching and allowed the authors to monitor how both the micellar shell and core structurally changed throughout the experiment.
The authors believe further investigation is required to understand the exact molecular process involved in agricultural sprays, in order to develop more efficient pesticide sprays in the future.
Click here to find out more about this reseach.
Reference
How does solubilisation of plant waxes into non-ionic surfactant micelles affect pesticide release
Hu, X., Gong, H., Li, Z., Ruane, S., Liu, H., Hollowell, P., Pambou, E., Bawn, C., King, S., Rogers, S., Ma, K., Li, P., Padia, F., Bell, G. and Lu, J.R. (2019). Journal of Colloid and Interface Science, 556, p.650-657
