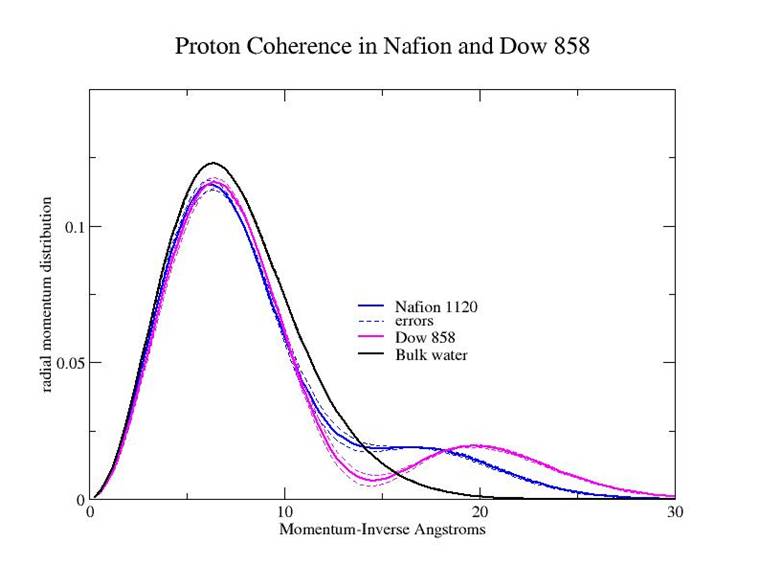
Momentum distribution measurements were made at room temperature on Nafion 1120, the commercial material, and Dow 858, the short side chain version of the polymer, at a water concentration in both cases of λ=14. The figure below shows the comparison of these two measurements with each other and with bulk water. It is evident that the quantum state of the protons in the Nafion materials is dramatically different from that of bulk water. The covalent bond has been replaced by a double well effective potential with, in both cases, a separation of the wells of ~0.2 Angstroms. There are more such delocalized protons in the Dow 858 material. The momentum distribution of the Dow material is very similar to the distribution of the protons in water confined in xerogel with pores of 23 Å diameter. The surface there is somewhat similar, it is irregular and there are hydroxyl groups projecting into the cavity. Since there is only one free proton for 14 water molecules, the hydrogen bond network must be strongly distorted by the presence of the surface to produce the observed momentum distributions. An attractive hypothesis is that the difference in conductivity is due to the difference in the quantum state of the proton, and that the conductivity in both materials is an example of coherent transport.
Proton momentum distribution in NAFION and similar compounds as measured on the VESUVIO spectrometer
View full-size image
Nafion is a sulfonated tetrafluorethyene copolymer used as an electrolyte in commercial fuel cells. A multiscale simulation of the structure is shown in the sidepanel. The hydrophobic backbones of the polymer enclose the water in a spongelike matrix, with the hydrophilic side chains containing the sulphonyl groups extending into the bulk water pockets. Conductivity in the material is due to protons donated from the sulphonyl groups of the polymer to the enclosed water matrix. Recently, a class of similar materials, with shorter side chains(DOW 858, DOW 840, DOW 1084) have been shown to have improved electrical and structural properties with regard to Nafion. The conductivity of two of these compared to Nafion, as a function of the fraction of water molecules to sulfonyl groups in the side panel.
Stephen Paddison (Univ. Tennessee), George Reiter (Univ. Houston), and Jerry Mayers (ISIS)
Research date: March 2010
Further Information
S. Paddison and J. A. Elliot, Molecular Modeling of the Short-Side-Chain Perfluorosulfonic Acid Membrane, J. Phys. Chem. A 109, 7583-7593 (2005)
