Halide perovskites are a series of materials that are used to make solar cells. Perovskite solar cells have developed rapidly over the last ten years to have high power conversion efficiency. The cells have the potential be made more cheaply than other competing technologies, but suffer from hysteresis that degrades their performance over time. Lead (Pb) halide perovskites have the general formula of APbX3, in which both the 'A' site cations and the 'X' site anions can be varied.
By mixing the 'A' site cations, scientists can tune the properties of the device, and enhance its stability. However, the reasons behind this improvement in stability were previously unknown.
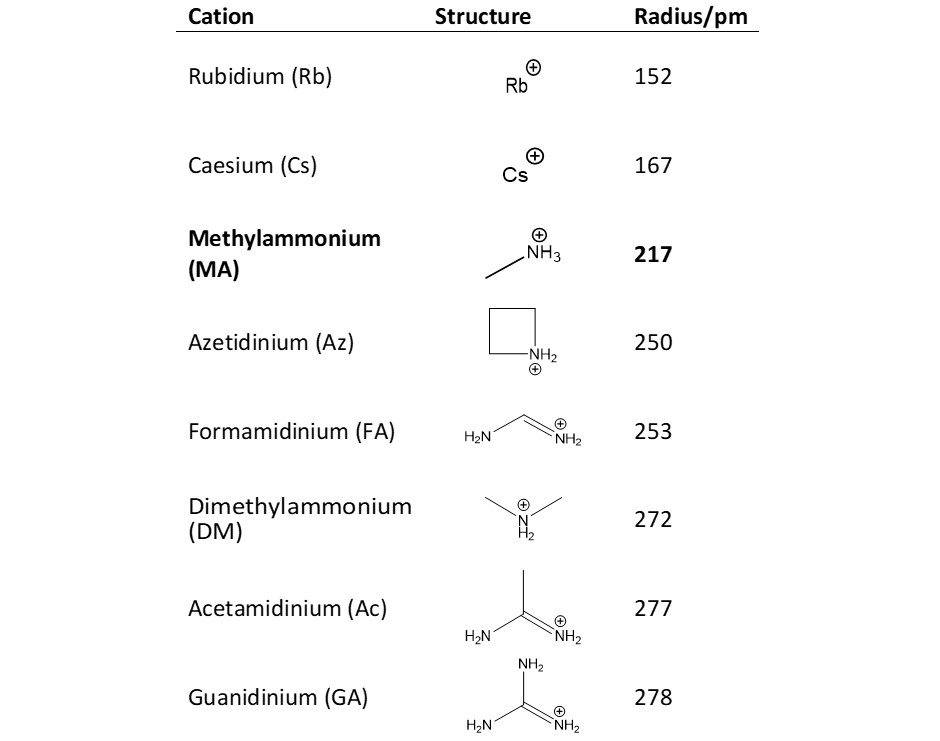
Researchers from the University of Bath have used ISIS to investigate a material where 'A' is methyl ammonium (MA) and 'X' is iodide (I‑): MAPbI3. Their study focussed on the iodide ion transport inside the material, by partially substituting the methyl ammonium (MA) with other cations. These cations ranged in size from small rubidium (Rb+) to large guanidinium (GA). They also studied how well solar cells of these substituted materials performed. The structures of the cations are shown in the table below: the largest size difference in to MA is GA.
Degradation in MAPbI3 has been linked to iodide ions moving through the material. Reducing this iodide ion diffusion could lead to a reduction in this degradation for this kind of solar cell material.
By using three techniques to build a comprehensive picture of the effect of changing the cation, they found that swapping just 5% of the MA for the larger GA ion strongly reduces iodide ion transport, and has implications for solar cell performance.
- Modelling found the paths that iodide ions would need to take through the material, showing that when a bigger cation is present and distorts the structure, the iodide has to follow a more difficult curved path.
- Muon Spin Relaxation (µSR) was used to detect ions moving inside the material. The µSR results agree well with the modelling results and clearly show that partially substituting A-site cations to MAPbI3 strongly reduces iodide ion transport.
- Impedance Spectroscopy showed how well ions could (or couldn't) move within the material at different temperatures. The results from these experiments also suggest that GA substitution strongly suppresses iodide ion diffusion.
Instrument scientist Peter Baker said: “It was interesting to extend the studies of ionic motion in battery materials we now do routinely to a completely different context and see such a dramatic effect for such a small change in the material composition."
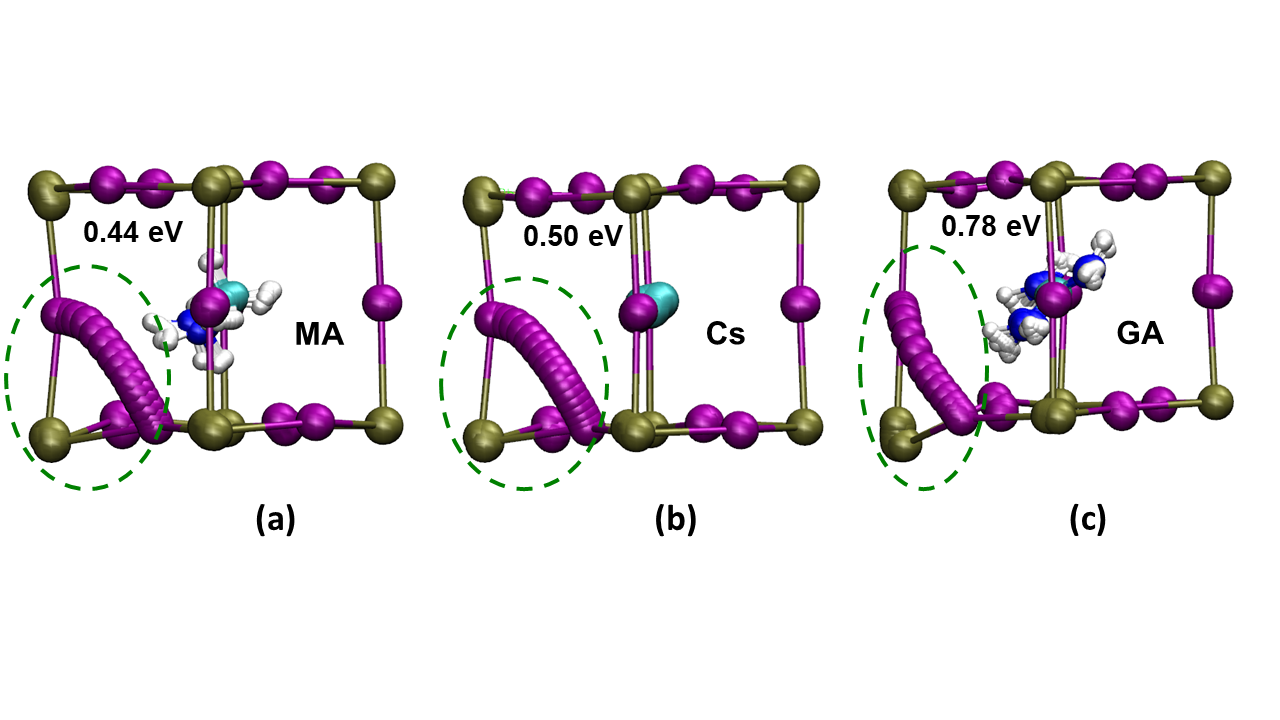
Image: Iodide ion transport paths and energetics. Ab initio simulations of the ion transport paths, the activation energies and the lattice ion displacements in (a) MAPbI3 (b) MA0.75Cs0.25PbI3 and (c) MA0.75GA0.25PbI3. (Key: Pb, green; I, purple.) Local lattice relaxations near the diffusion path are highlighted by green circles, showing greater structural distortion in the GA-substituted material.
The results from all three techniques showed strong agreement: all the cation substitutions increased the activation energy for iodide ion transport relative to pure MAPbI3. This combined study has enhanced the fundamental understanding of mixed-cation perovskites, and provides a design strategy for reducing iodide ion transport that has important implications for improving solar cell performance.
“By using three different techniques to study perovskite materials we were able to really understand the effect of cation substitution. The information we obtained from muon spectroscopy was fundamental to allow us to build a model of what was happening inside the cells," said researcher Petra Cameron. “This was our first time carrying out measurements at ISIS, and the muon spectroscopy has really helped us to build up an understanding of ion motion in solar perovskite materials".
Further information
The full paper can be viewed online at DOI: 10.1039/C9EE00476A, Energy Environ. Sci., 2019, 12, 2264-2272
For more science highlights from MuSR, click here.
