A team, led by scientists at the Universities of Birmingham and Nottingham, have used small-angle neutron scattering to investigate the cooperative interactions of the multifunctional ParB protein, KorB, required for plasmid DNA partitioning and transcriptional repression in bacteria, with operator DNA and the repressor KorA. Their results, confirmed by the LOQ instrument at the ISIS Neutron and Muon source, reveal that the positive co-operativity between KorB, KorA and DNA results from conformational restriction of KorB on binding each partner, while maintaining intrinsic disorder.
Transcriptional repression represents one of the most ancient and conserved mechanisms of regulating gene expression throughout all forms of life and comes in many different forms. Negative regulatory pathways can limit the production and activity of activator proteins, whilst DNA-binding transcription repressors can actively block transcription, with complexity varying between Bacteria, Archaea and Eukarya to meet the differing structural and functional demands of organisms belonging to each domain of life. In Bacteria, transcriptional repression pathways involve the direct action of DNA-binding repressor proteins on the transcriptional machinery, for example at promoter regions, to block RNA polymerase activity, commonly during the processes of initiation and elongation.
KorB, from the low copy-number IncP1 plasmid RK2, is an example of such a protein required for transcriptional repression in Gram Negative bacteria, such as Escherichia coli. One of the best- characterised of the ParB proteins, KorB binds specifically to several sites on the plasmid DNA and to the RK2 homologue of ParA, IncC, stimulating the latter's ATPase activity. The protein also plays a role in plasmid partitioning and hence the stable transmission of self-replicating, extrachromosomal DNA during bacterial cell division.
|
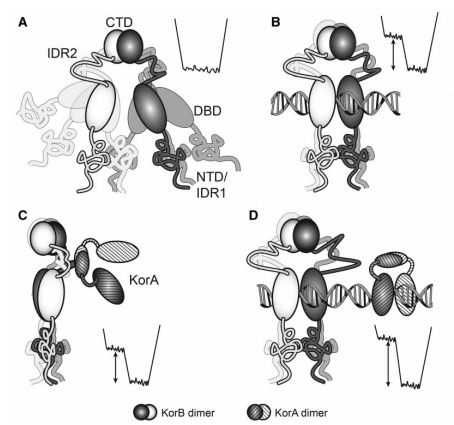
Cartoon of KorB and its complexes with a sketch of the co-operative binding process as applied to intrinsically disordered proteins: Image credit: E. I. Hyde,et. al. "Intrinsic disorder in the partitioning protein KorB persists after co-operative com plex formation with operator DNA and KorA," Biochemical Journal, no. 474, pp. 3121-3135, 2017. DOI: 10.1042/BCJ20170281 . |
In this pioneering experiment, the team, jointly led by Dr. Eva Hyde at the University of Birmingham and ISIS Senior Molecular Biology and Neutron Fellow, Dr David J Scott, investigated KorB in order to understand how the protein mediates both its DNA binding and its co-operative mode of action with KorA. Small-angle neutron scattering (SANS) taken at both the Institut Laue Langevin in Grenoble and at ISIS Neutron and Muon Source, along with circular dichroism from B23 beamline at Diamond, and NMR spectroscopy at the Henry Wellcome NMR Centre at Birmingham, were used to selectively probe the conformation of KorB in binary and ternary complexes with KorA and DNA. What the study reveals is that the bound KorB protein remains disordered, with a mobile C-terminal domain and whilst there are no changes in the secondary structure, there are increases in the radius of gyration on complex formation. By comparing wild-type KorB with an N-terminal deletion mutant, a model of the ensemble average distances between the domains when bound to DNA was also constructed.
The SANS technique employed in this experiment is particularly useful for studying macromolecular complexes as different nuclei, particularly deuterons and protons, scatter neutrons with different phase and intensity, meaning that DNA and proteins can be elucidated within a complex. The LOQ instrument at ISIS Neutron and Muon Source - the most successful time-of-flight SANS instrument in the world - can be used to investigate the shape and size of large molecules, small particles or porous materials with dimensions in the range of 1-100 nm. The instrument itself is relatively simple, consisting of an 11-metre evacuated beamline down which neutrons fly towards a sample. Following scattering, the neutrons hit a fixed 2D detector 4 metres away, which can detect the positions and times of arrival of the neutrons. Information on the nanostructure of the sample can then be deferred by analysing the resulting pattern.
“The beauty of the SANS technique is that we can look at the shape of each component in the complex individually. The advance in this study is that we have looked at a complex with three components, 2 different proteins and a DNA molecule, with contrast matching, rather than just two components" said Dr Hyde “One complication is that the KorB protein has long segments of structure that are disordered, so it has a range of shapes in solution, not just one fixed shape. This, and the size of the complex, means that SANS is the best method to study it."
Dr David J. Scott, University of Nottingham
Using SANS and contrast matching, the results pub lished in Biochemical Journal have shown that the binding of DNA and/or KorA causes a change in the 3D shape of KorB, to favour longer, more extended conformers on complex formation. Such measurements, along with the known crystal structures of the DNA-binding and C-terminal domains, allow a general model of the ensemble average distances between the domains in the DNA complex to be constructed.
“The KorB system has been a fascinating set of macromolecules to work on" said Dr. Scott. “We started this work in 2006, thinking that the project would be finished in less than a year, however, as we investigated further the intricate subtleties of the system started to become apparent. KorB belongs to a set of proteins, intrinsically disordered proteins, that have sections that do not fold to a unique three- dimensional structure, yet the proteins are able to carry out specific biological functions. As such they are of great interest in Structural Biology where the paradigm is that unique structure defines specific function. The SANS technique allowed us to probe the conformation of KorB in the presence of both DNA and the partner protein KorA, and hence show that the protein has the same amount of disorder, irrespective of what it is bound to. Circular dichroism studies on beamline B23 at Diamond also confirmed these findings showing the power of the complementary methods available on-site at the RAL for researchers. It was also another excellent example of collaboration between the facilities on-site."
Dr David J. Scott, University of Nottingham
The research was supported by the Science and Technology Facilities Council (U.K.) in collaboration with the Wellcome Trust and the EU. The full publication is available to view in Biochemical Journal
For further information about the research, please contact Dr David J. Scott and Dr. Eva Hyde (david.scott@nottingham.ac.uk / e.i.hyde@bham.ac.uk).
Further Information
E. I. Hyde, P. Callow, K. Rajasekar, P. Timmins, T. R. Patel, G. Siligardi, R. Hussain, S. A. White, C. Thomas and D. J. Scott, "Intrinsic disorder in the partitioning protein KorB persists after co-operative complex formation with operator DNA and KorA," Biochemical Journal, no. 474, pp. 3121-3135, 2017. DOI: 10.1042/BCJ20170281 .
Further details on the LOQ instrument can be found here.
