But what happens when we want to save some of this feast for later? With the global consumption of electricity set to increase by over 60% from 2011 to 2030 , it’s clear we need to make the most of the energy we produce and so a balance between supply and demand is vital.
The lithium-ion batteries, powering our smartphones, home appliances and electric vehicles, are a triumph of modern technology and offer an efficient and sustainable energy storage solution. In fact one of the largest battery arrays in the world, found in China, utilises a football pitch of lithium-iron-phosphate (LiFePO4) batteries to store energy generated from wind and solar power. However, current batteries are limited in terms of their performance and therefore new synthetic routes to battery materials and a better understanding of the mechanisms driving these batteries are needed to improve them.
Scientists show muons’ ability to explore lithium diffusion in microwave-made nanosized battery materials.
A group led by Dr Serena Corr, Glasgow University, have synthesised nanoparticles of the battery material, LiFePO4, using a microwave synthesis method and investigated the diffusion of lithium in this nanostructure for the first time using muons. By looking at how these battery materials work at the atomic scale they hope to develop better battery materials in the future. The research has been published in the Journal of Materials Chemistry A.
“LiFePO4 has attracted a lot of attention as it presents an economical and non-toxic option for a lithium-ion battery material”, explains Dr Serena Corr. ‘It has a high charge density, good cyclability and is complementary to most conventional polymer electrolytes.”
Lithium-ion batteries usually consist of two electrodes an anode and a cathode – and lithium ions flow between them during charge and discharge. Depending on the type of material and synthesis route used to make a cathode, the costs, voltage, energy density, lifetime and safety of a lithium-ion battery can change dramatically. So to build better batteries a deeper understanding of the inner workings of battery materials is required.
LiFePO4 is an example of a cathode material and its crystal structure is indicated in the figure below. The lithium ions shown in green exist in channels which they move into and out of during charge and discharge cycles. This lithium diffusion rate determines how quickly a battery can be charged and discharged and the team have used muons to measure this rate.
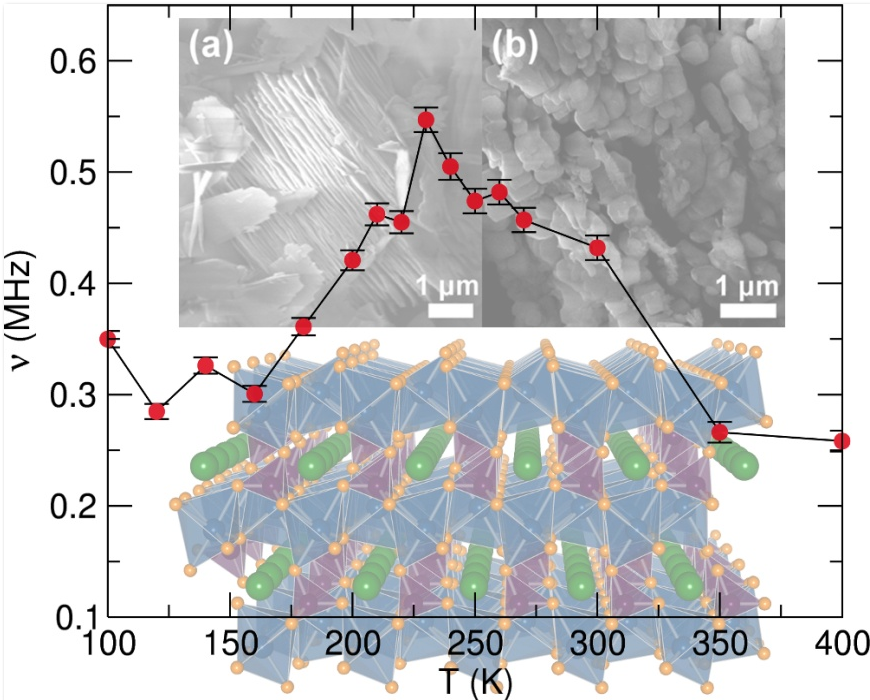
Top (a), (b): Electron microscopy measurements of LiFePO4 nanostructures prepared by microwave synthesis; Bottom: Structure of LiFePO4, with iron atoms in blue, phosphorus in purple, oxygen in orange and lithium in green. Overlay: Temperature dependence of the fluctuation rate from fitting muon data, from which the lithium diffusion coefficient and activation energy can be determined.
Serena’s group are interested in making nanoparticles of battery materials, “when you get down to very small particle sizes, the lithium diffusion distance is much shorter. These particles also have a larger surface area available compared to bulk materials so there is more chance to be able to insert or remove lithium from the cathode. So with these smaller sized particles, you have an opportunity to improve the electrochemical performance of LiFePO4.”
“One of the things that is clear from the literature is just the vast difference in the numbers obtained for the lithium diffusion coefficient in this material (LiFePO4) which can give you an idea of the rate that lithium is moving through your structure. If you look at the structure you’ll notice the only way the lithium can get in and out is through these channels. What’s more, this picture shows the ideal structure, whereas in reality it’s not perfect. Sometimes the iron atoms sit where the lithium atoms should be, blocking the channel. This could be due to the way the material is prepared or the timescales in which you probe the diffusion and all of these can combine to create an interesting problem to solve.”
The team used the Emu instrument at ISIS to investigate the diffusion of lithium within this nanostructure using muon spectroscopy. “We have demonstrated that muon spectroscopy measurements can be a powerful probe of lithium diffusion in nanoparticles. Previous studies have used muons to probe the Li diffusion in bulk LiFePO4; however, this is the first time we have observed thermally activated lithium hopping in nanoparticles of the material. What we’ve shown is that we get very similar lithium diffusion coefficients for these nanoparticles which, in itself, is quite an important result because it demonstrates the technique to be extremely reliable and independent of how the sample is prepared. The results we obtained are also very close to theoretical predictions which strengthens the claim that muon spectroscopy is excellent for probing this lithium diffusion on short length scales.”
So why use microwaves?
“We’re trying to look at greener and more efficient ways of making nanomaterials. Traditionally if you want to make a bulk material like LiFePO4 you grind your solid starting materials together, press them into a pellet and heat in a furnace at very high temperatures for a couple of days or even a week. This takes a long time and is costly in the long run but you need these high temperatures to overcome the associated lattice energies.”
With microwaves however, the team have been able to take their starting powders and disperse them in a solvent with a dipole moment. The solvents chosen here interact strongly with the incoming microwaves, absorbing this microwave energy and converting it to heat through the dielectric heating effect. The benefit of targeting the solvent is everything is heated at the same rate, promoting the nucleation and growth of nanoparticles with the same size and shape at much quicker reaction times.
In fact the team have managed to bring the reaction time down to as short at 15 minutes. “We’re still looking to get it down even more” explains Serena. “Instead of having two or three starting materials, we are now looking to design new precursor materials where everything we want is in a single precursor, so that the metals don’t have to travel very far to react with each other, which should make our reaction times extremely short.”
The group are interested in using multiple methods to characterise their materials from bulk methods to x-ray diffraction to muon spectroscopy. Recently they have also been doing experiments on Polaris; “On Polaris we can get information about the local structure of the materials. So between the muon experiments, where we learn about lithium diffusion, and these local structure experiments, where we actually learn about the lithium content of our materials and the nature of any defects in the materials we make, we’ll be able to put the data together to get a really intimate understanding of what’s going on. Once we’ve done that we can further optimise our synthesis and try to work out a synthetic route to give the least number of defects.”
The group plan to return to ISIS for more muon experiments soon where they hope to enhance the electrochemical performance of their materials by replacing some of the iron atoms with other metals like manganese.
So what about the future of lithium battery materials?
“Lithium-ion batteries have gone from a stage of discovery and development to being employed in real devices like portable electronics and electric vehicles in little over 30 years. This makes working on energy storage materials like these sorts of compounds very exciting - in the next ten years who knows what’s going to happen! But I’m sure that having access to the muon source and the high resolution facilities at ISIS, the things we find out now will help us to decide our future research direction.”
Felice Laake
Research date: April 2014
Further Information
For more information please contact Dr Serena Corr
References
[This research has been published in the Journal of Materials Chemistry A.
1] Advanced Energy Storage Systems Market by Technology (Pumped Hydro, Compressed Air, Batteries, Flywheels, Supercapacitors), By Applications (Grid Storage & Transportation), & Geography - Global Trends & Forecast to 2018. Markets and Markets. 2013.
