Their results have been recently published in Nature Communications.
Ceria (CeO2) is a stable, pale yellow oxide of the rare earth metal cerium. Despite the name, cerium is fairly common (almost as abundant as copper in the Earth’s crust) and its oxides have many applications. Ceria is used as an oxidizing species in catalytic converters to help remove toxic gases such as carbon monoxide, as an electrolyte material in solid-oxide fuel cells, in the chemical-mechanical polishing of surfaces, and even in the walls of self-cleaning ovens.
The use of ceria as an oxidation catalyst comes from the unique cerium (III/IV) redox chemistry (i.e. interconversion between Ce3+ and Ce4+). This, alongside its cubic fluorite lattice structure, makes ceria a responsive oxygen buffer through the storage and release of oxygen. Ceria nanoparticle catalysts are even more active due to an enhanced surface area to volume ratio and superior oxygen storage capacity, and catalytic activity can be further enhanced by controlling the morphology. Consequently, nanostructured ceria can be used to greatly enhance catalytic activities for a number of important reactions, but many current preparations require intensive conditions, such as high temperatures, long reaction times, or corrosive or toxic additives.
In their Nature Comms paper, Hammond et al describe the novel synthesis method of nanostructured ceria using the Deep Eutectic Solvent (DES) reline, allowing for morphology and porosity control via a less energy-intensive route than previous synthetic methods.
DESs are an extended class of ionic liquids with special properties, comprising a mixture of compounds where the melting point is much lower than the individual components. They are relatively non-toxic, ‘green solvents’ that can be tailored for specific applications. Reline, a DES composed of urea and choline chloride, is particularly useful since it is quite cheap, tractable and biodegradable.
Using the SANDALS wide Q-range neutron diffractometer at ISIS, Hammond and colleagues investigated the mechanism of the novel nanoceria synthesis at a molecular scale. They had previously determined the structure of bulk reline using neutron diffraction, showing a complex hydrogen-bonding network between species with some preferred choline-chloride and urea-chloride bonding. In this study, they were able to determine the structure of the solvated cerium species in reline that underpins the reaction mechanism. Diffraction data revealed that the cerium precursor salt is readily integrated into the DES matrix of reline by hydrogen bonding, and that reline forms a pre-structured liquid where reactive components are brought together, thus acting as a latent supramolecular catalyst.
“The wide Q-range neutron diffraction technique is absolutely invaluable for studies such as these, because we are able to isotope-substitute hydrogen for deuterium in the samples to get sets of scattering data that appear completely different at first glance, but correspond with the same overall structure. This lets us see the most favoured interactions in the complicated and disordered liquid DES ‘alphabet soup’, where the various DES components are in constant motion, making and breaking bonds between molecules millions of times per second. No other technique is currently capable of giving such accurate, direct measurements of the structure of a liquid.”
Hammond and colleagues went on to discover that by addition of controlled amounts of water, the morphology, porosity and consequently catalytic activity of the ceria materials could be finely tuned. By adding water, they were able to tune the reaction to create highly active ceria nanorods and nanowires under milder conditions then previously reported.
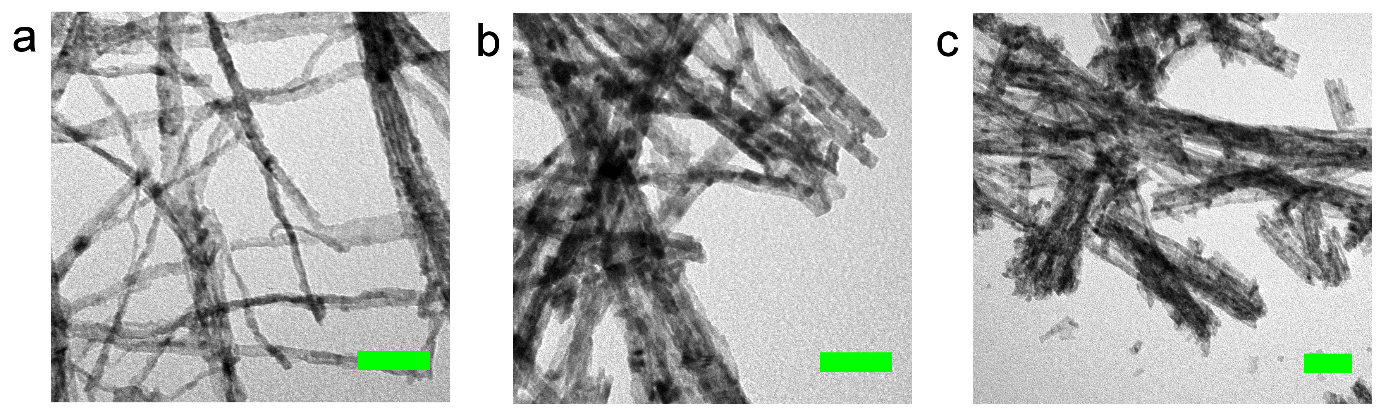
TEM images of the ceria nanorods and nanowires prepared at (a) 100 ˚C, (b) 140 ˚C and (c) 180 ˚C, using a reline DES diluted with 10 mole equivalents of water.
View full-size image
“Using DESs for nanoceria synthesis is therefore a step forward in both environmental and economic terms. This work presents a new, milder, greener route using cheap, non-toxic and biodegradable precursors alongside water as a structure-modulating agent, to synthesize thermally stable ceria nanostructures with enhanced catalytic activity. We hope that this solvothermal method using DESs and water will prove useful not just for the synthesis of ceria, but also for more environmentally-friendly preparations of other useful metal oxide nanoparticles.”
Preeti Kaur
The research paper published in Nature Communications can be found
here.
