A comprehensive study, led by the Knowles research group at the University of Birmingham, of the membranes found in Gram-negative bacteria has shown that the protein MlaC, previously thought to move lipids from outside to inside the cell, actually does the opposite: exporting the lipids from the internal membrane towards the outer cell membrane.
Rising antimicrobial resistance, particularly in difficult-to-treat Gram-negative infections, is rapidly becoming one of the biggest challenges facing the 21st century. A key research priority now lies in identifying interactions between molecules inside Gram-negative bacteria that could be exploited as novel drug targets. Of particular interest are the interactions involved in the synthesis of the cell outer membrane.
The outer membrane of Gram-negative bacteria is made up of a double membrane. The inner membrane is composed of two layers of phospholipids (labelled PL below), and the outer is asymmetrical with phospholipids on the inside and lipopolysaccharide (LPS) on the outside. The structure of this asymmetric membrane serves as a formidable barrier against environmental stress, antibiotics and immune factors.
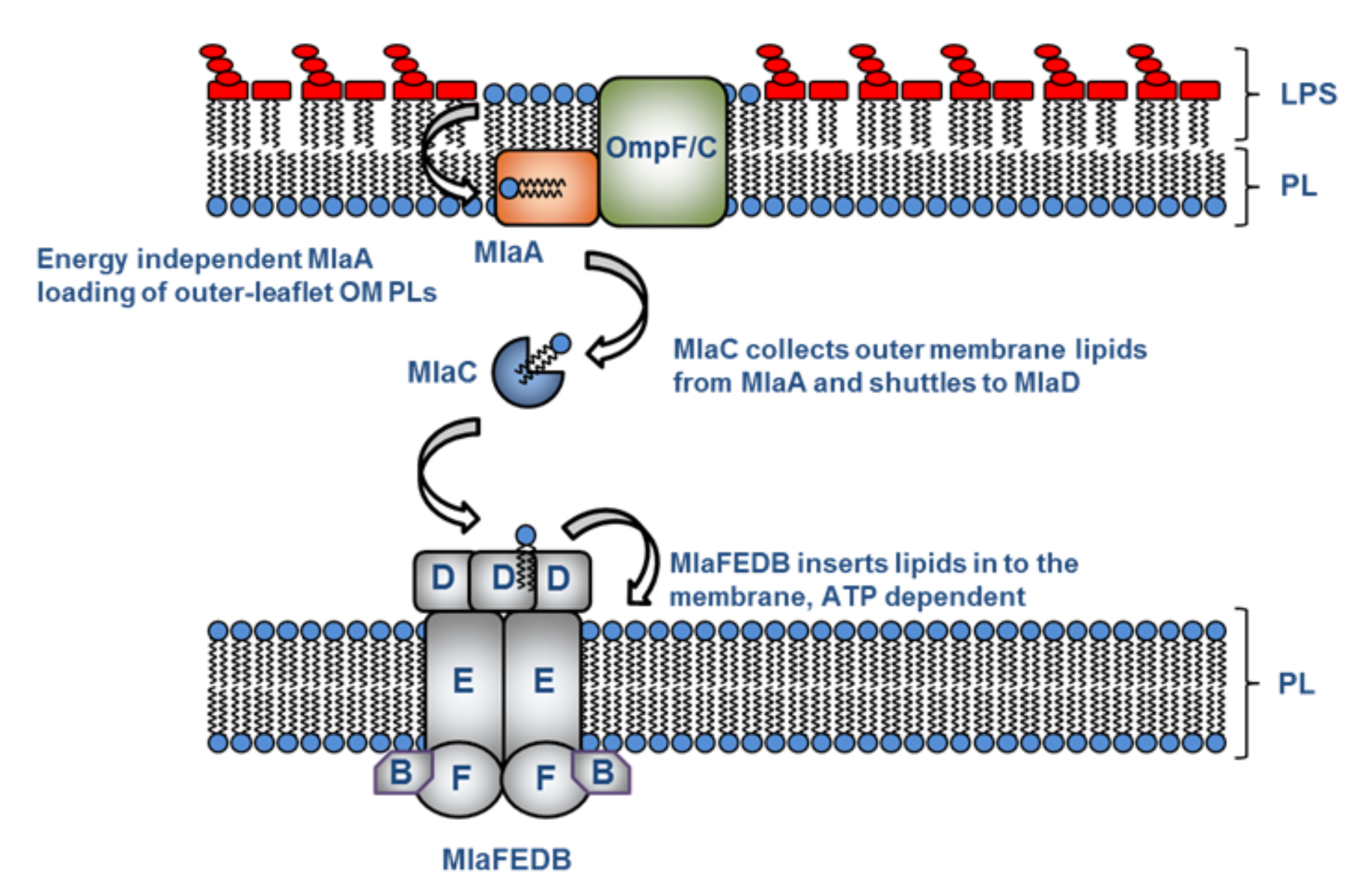
As shown in the figure above, it was thought that there was a pathway where the phospholipids from the outer membrane were moved to the inner membrane to maintain this asymmetry. The pathway components include a lipoprotein in the outer membrane (MlaA), which is believed to extract phospholipids; a soluble component in the periplasm between the membranes (MlaC) thought to transport phospholipids between the membranes; and an inner membrane enzyme (MlaFEDB) that is assumed to accept and insert phospholipids into the inner membrane using ATP as its power source.
In this study, the researchers focussed on the interaction between the periplasmic MlaC and the MlaFEDB complex on the inner membrane. They found that the pathway does not operate as previously thought, and that the process is much more complex.
The group carried out specifically designed quartz crystal microbalance and infrared based experiments at the Biology Lab at ISIS and saw that, when MlaC was flowed over MlaFEDB within the membrane, phospholipids were actually removed from the inner membrane, not added. This was the case even when the MlaFEDB complex lacked its energy source, ATP, sparking the suggestion that it may have another, yet unknown, function within the cell.
These results were supported by neutron reflectivity measurements on INTER, and a huge range of techniques from other facilities, including the Diamond Light Source.
This complex study provides, for the first time, evidence of a pathway involved in transporting phospholipids towards the outer membrane of a bacterial cell.
Further information
The full publication can be found at Nature Microbiology.
Other science highlights using INTER can be viewed here.
