Scientists from Istituto Italiano di Tecnologia, Politecnico di Milano and ISIS have published a study on a novel amphiphilic molecule, called ZIAPIN2, that enters a cell membrane rapidly with virtually no energetic barrier to overcome and, importantly, its properties can be altered by light.
Amphiphilic molecules have both water-loving and fat-loving properties, and therefore have the potential to modulate the properties of cell membranes. Although sometimes considered like a barrier, cell membranes are fluid, meaning the molecules embedded within them can move around.
The researchers mapped out a wide range of motions in both model membranes and intact cells using neutron scattering on the IRIS instrument. They observed the effect of ZIAPIN2 and the vehicle solvent dimethyl sulfoxide (DMSO) on these movements within the picosecond timeframe.
The results, published in Langmuir, showed cell membranes became softer and more flexible after ZIAPIN2 insertion. When the cells were illuminated, the researchers also observed a process known as photo-isomerisation causing a reduction in membrane thickness. This led to a subsequent change in the cell membrane potential.
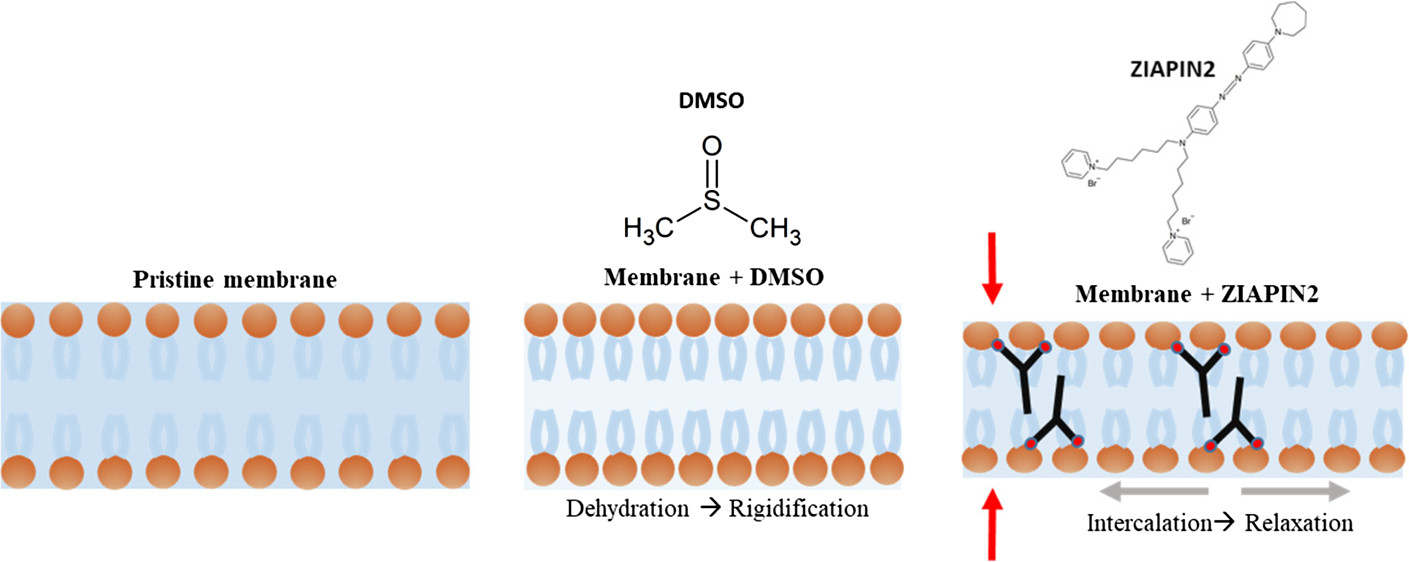 Pictorial representation of the dynamical scenario caused by DMSO and ZIAPIN2 addition to the POPC liposomes. Langmuir 2020, 36, 39, 11517-11527
Pictorial representation of the dynamical scenario caused by DMSO and ZIAPIN2 addition to the POPC liposomes. Langmuir 2020, 36, 39, 11517-11527
“ZIAPIN2 photo-isomerisation allows cell activity to be controlled via light illumination," says Dr Paternò, one of the researchers working on this project. For example, a neuron could be made to fire on demand.
In practice, DMSO is currently used to increase cell membrane permeability. This study demonstrates that combining DMSO and ZIAPIN2 leads to much more complex dynamics.
There are multiple future avenues for this research, explains Dr Paternò; “First, we are trying to gain insight into the biophysics underpinning the photo-modulation of signals. Second, we are studying different molecules that can photo-stimulate cells by another physical mechanism, for instance by displacing charges."
Future experiments would be likely to use neutron scattering in combination with light studies. The hope is that gaining insight into how these molecules affect cell membrane behaviour could lead to the development of membrane-targeted antibiotics and antiviral drugs.
Further Information
The full paper can be found at DOI: 10.1021/acs.langmuir.0c01846
