As you may have noticed from the formation of snowflakes, water does not freeze naturally into cubes, but actually into a hexagonal structure. By developing a novel synthesis method, a group of researchers led by Professor Lorenzo Ulivi from L'Istituto di Fisica Applicata “Nello Carrara", part of the Consiglio Nazionale delle Ricerche (CNR) in Italy, were able to synthesis pure cubic ice on HRPD and characterise some of its properties.
At a microscopic level, water molecules form hydrogen bonds to their four nearest neighbours. Three of these produce a sheet of hexagonal rings (as shown below), which is slightly puckered, with the fourth joining sheets when they stack one on top of the other.
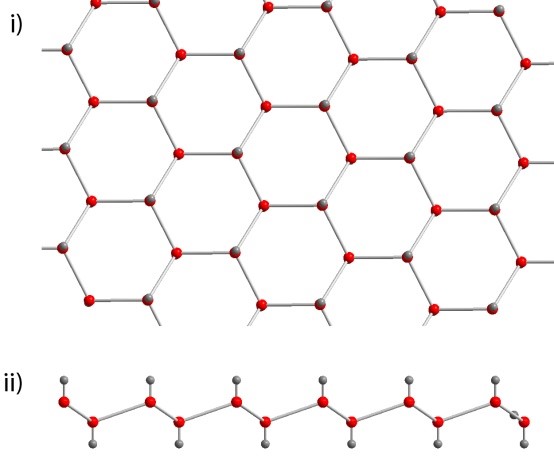
The layers of hexagonal rings found in ice, viewed from above (top) and along the edge of the layer (bottom).
These sheets can be stacked in two different ways. In the first, the sheets stack in alternating layers, where one is the original sheet (A) and the other is its mirror image (B). This stacking sequence (A-B-A-B…) generates a hexagonal-close-packed array of water molecules in three-dimensional space and the overall symmetry of the crystal is hexagonal. We call this form of ice, ice Ih (where I is the Roman numeral 1), and it is the variety that we make in domestic freezers, and that forms all the Earth's glaciers and ice sheets.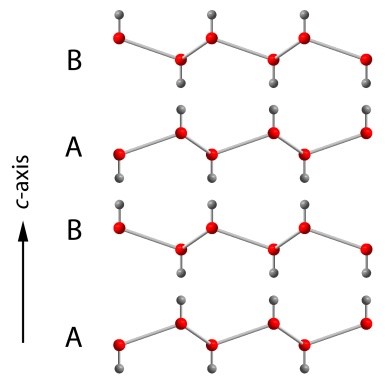
Stacking sequence of the layers in hexagonal ice Ih. Red spheres are oxygen atoms and the grey spheres are hydrogen atoms.
In the second formation, the sheets are stacked as A-B-C – A-B-C – A-B-C etc., where sheet B is a copy of sheet A but moved sideways by 1/3 the width of a hexagonal ring and sheet C is a copy moved across by 2/3 of a ring (as shown below). The next shift would be 3/3, or 1 ring, so the fourth layer is simply repeating the first (A). This molecular arrangement is known as cubic-close packing and is a symmetrically cubic crystal, known as ice Ic.
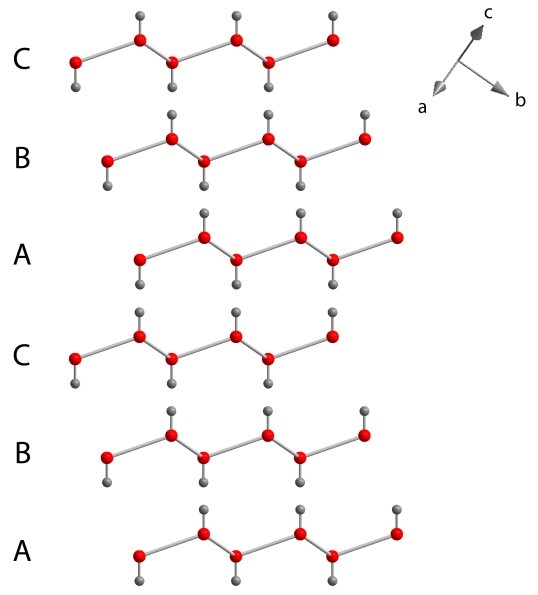
Stacking sequence of the layers in cubic ice Ic.
This second, cubic, form of ice is much more difficult to make. It usually forms by vapour deposition of water at very low temperatures, and it is likely to form in the Earth's polar stratosphere. In the laboratory, the most straightforward synthesis method is from high-pressure forms of ice: high-pressure ice brought back to atmospheric pressure will form cubic ice on warming, before ending up as ordinary hexagonal ice.
The stacking sequences described above can be mixed together. It's possible to have combinations of hexagonal and cubic layer repeats in the same crystal, which don't exhibit any long range order. In fact, all of the so-called cubic ice made to date is actually this form of stacking-disordered ice. Various methods have tried to increase the 'cubicity' but none has so far exceeded about 80%. Until now!
This work, done on HRPD, details a novel synthesis method that allows the formation of 100% cubic ice, with no measurable hexagonal stacking sequences. The group started with a high-pressure form of hydrogen hydrate, a crystal in which there is a framework of water molecules arranged in such a way as to create cavities in which the hydrogen is located. A dynamic vacuum removed the hydrogen from the structure of the hydrate crystal on the HRPD beamline, which formed a very low-density form of ice called ice XVII.
This polymorph of ice is characterised by a very open structure and purely 5-sided rings of water molecules. On warming, it transforms first to pure cubic ice and then to pure hexagonal ice. Their work, including additional measurements on D20 at the ILL and Raman spectroscopy in IFAC has just been published in Nature Materials.
This new synthesis method now allows bulk preparation of pure cubic ice so that details of its thermodynamics, thermal expansion and possible proton ordering can be explored in more detail and compared with the well-known anomalous properties of hexagonal ice.
Further reading
Full paper at: https://www.nature.com/articles/s41563-020-0606-y
Previous work on ice XVII: del Rosso et al., 2016a, 2016b.
