Using neutron diffraction on the PEARL instrument, ISIS scientists have investigated the high-pressure structural behaviour of the layered perovskite materials La2NiO4+δ and Pr2NiO4+δ. By comparing the effects of physical compression and chemical doping, they were able to show that both techniques influence the material's structure in a similar way.
The authors suggest that a high-pressure technique could form the basis for preselecting the level of chemical doping required to tune the desired material properties. This has important implications for how materials may be synthesised with properties tuned for new applications, as it has the potential to be a more precise process, and less intensive than synthesising multiple samples.
Both La2NiO4+δ and Pr2NiO4+δ have a structure that consists of perovskite-like sheets of corner-shared NiO6 octahedra that are separated by rock-salt like layers, as shown in the diagram below. Materials with this structure show interesting properties; La2NiO4 shows a temperature driven semi-conductor to metal transition, making it potentially highly functional.
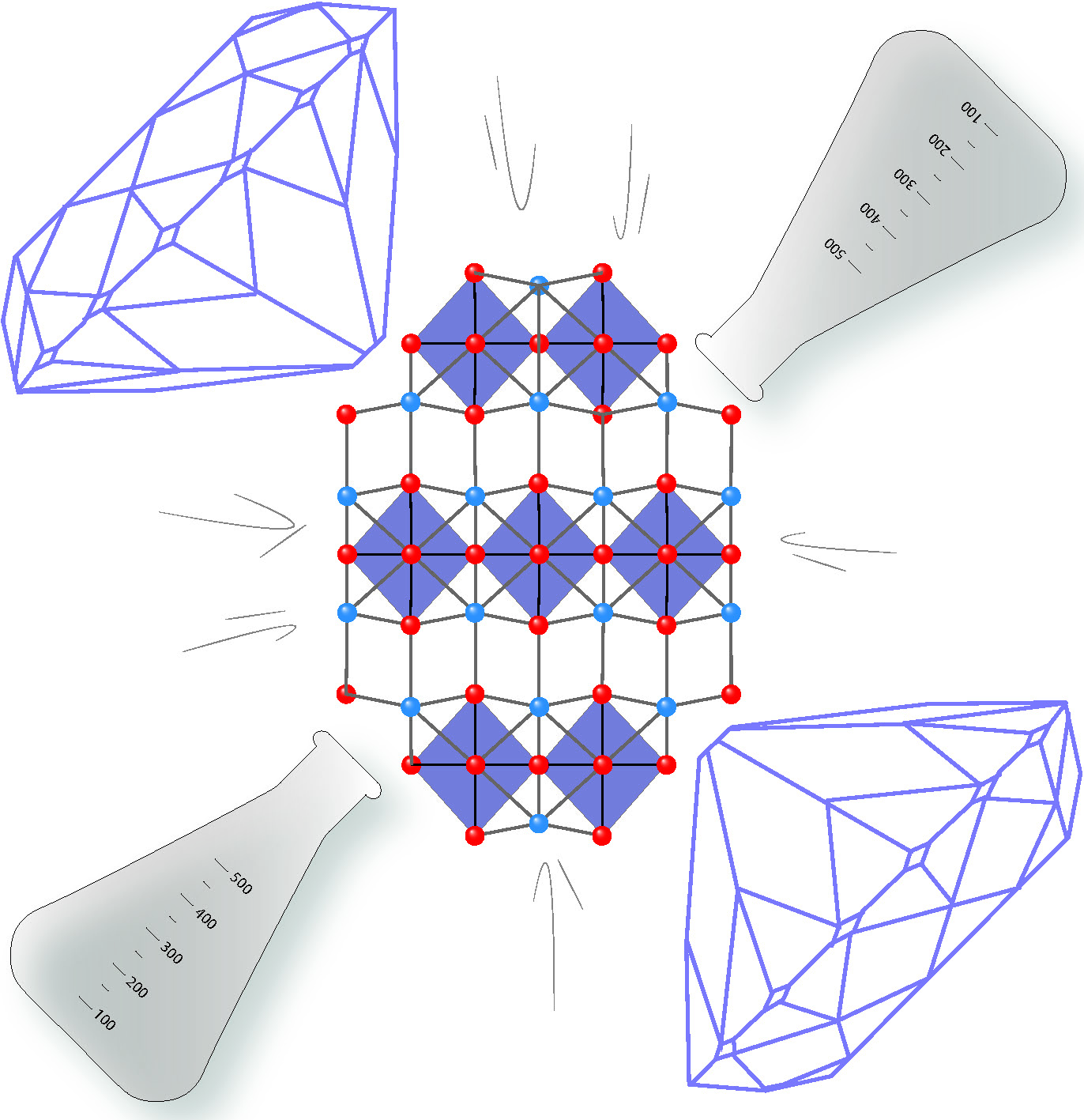
Structure of the layered-perovskite X2NiO4 (X = La or Pr) looking down the b-axis. The NiO6 octahedra are shown in purple. The offset O and X atoms along the c-axis are clearly seen as a zig-zag across the structure. Application of high pressure changes both the length and angles of bonds which can also be achieved by chemical means.
By applying pressure to materials, researchers are able to control the distances between the atoms inside them. The change in the atomic positions also changes the properties of the material, forming new structures with potentially desirable traits. Another method of creating distortions to the structure of a material such as La2−xPrxNiO4 is chemical doping, where the value of x is varied.
Understanding the effects of both types of distortion, and whether they can be used to create the same change in properties has implications for materials design. Applying certain pressure conditions can be used to finely tune a property, which could then be re-created using chemical doping, potentially reducing the need for extensive and synthesis of materials across a whole solid solution range and hence permitting a more targeted approach to synthesis in the laboratory.
In this study, published in Dalton Transactions, the materials under study were La2NiO4+δ and Pr2NiO4+δ. The researchers found that the mechanism of compression was different for the two materials in the way the NiO6 octahedra and La/PrO9 polyhedra behave. Compression of La2NiO4+δ resulted in a small average increase in NiO6 polyhedral volume, but a measurable decrease in LaO9 polyhedral volume. However, compressing Pr2NiO4+δ noticeably reduced the volume of both the NiO6 and PrO9 polyhedra.
When considering chemical pressure, increasing x in both La2−xPrxNiO4 and Pr2−xNdxNiO4 causes a measurable decrease the volume of both NiO6 and La/PrO9 polyhedra. This shows that, for some materials, the application of pressure can predict the effect of chemical doping. However, the difference in oxidation state on doping also needs to be taken into account.
This study sets the groundwork to show that it is possible, in the right chemical system, to use physical pressure measurements to inform synthesis routes to new materials that have specific desired physical or structural properties.
Further information
The full paper can be found online at DOI: 10.1039/D0DT02155E
