Investigating battery performance and lifetime at IMAT
10 Nov 2020 - Rosie de Laune
Leveraging the unique interaction of neutrons and lithium, researchers have been able to build a unique picture of what’s happening inside a lithium battery as it undergoes cycling. Lead researcher on the experiment, Ralf Ziesche, has just finished his PhD as part of an ISIS Facility Development Studentship, joint with Professor Paul Shearing at UCL. During his PhD, Ralf worked on the neutron imaging and diffraction instrument IMAT at ISIS developing neutron imaging techniques for electrochemical devices such as batteries and fuel cells.

X-rays are commonly used for imaging these types of devices but, due to the nature of X-rays, which interact with the electron cloud rather than the nucleus, lithium ions are largely transparent using this technique. This makes looking into details of battery materials containing lithium especially challenging.
However, neutrons are very sensitive to lithium, and to any hydrogen present, and consequently neutrons imaging uncovers provides unique insight of what goes on inside a battery during charging and discharging. “Neutron imaging at high spatial and temporal resolution is an emerging technique” explains Ralf; “but it provides the power to visualise the lithium inside a battery, making this the perfect complementary technique to X-rays.”
Neutron imaging at high spatial and temporal resolution is an emerging technique
Ralf Ziesche
His two-part study, published in the Journal of The Electrochemical Society, combines their work on IMAT with X-ray imaging to quantify the lithium remaining in the anode during discharge of a LS 14250 ½ AA sized commercial Li/SOCl2 battery cell designed for low-rate applications such as backup batteries in computers or alarm systems.
Thanks to the use of a ‘golden-ratio’ scanning method, Ralf and his collaborators were able to collect a sequence of tomographies and build-up a four dimensional image of the inside of a battery whilst it was being discharged. They were able to track the discharging by looking at the movement of the lithium ions, removed from the Li-metal anode and diffusing into the thionyl chloride cathode. Effects such as lithium diffusion blocking which diminishes the maximal cell capacity were investigated and correlated with the electrochemical results.
Using the combination of neutrons and X-rays, they were able to identify all battery cell components that are difficult to distinguish using one of the methods alone. Their imaging technique also enabled them to see exactly where problems occur: they saw that lithium diffusion was being blocked by the LiCl protection layer, an insight that is extremely valuable for future battery design.
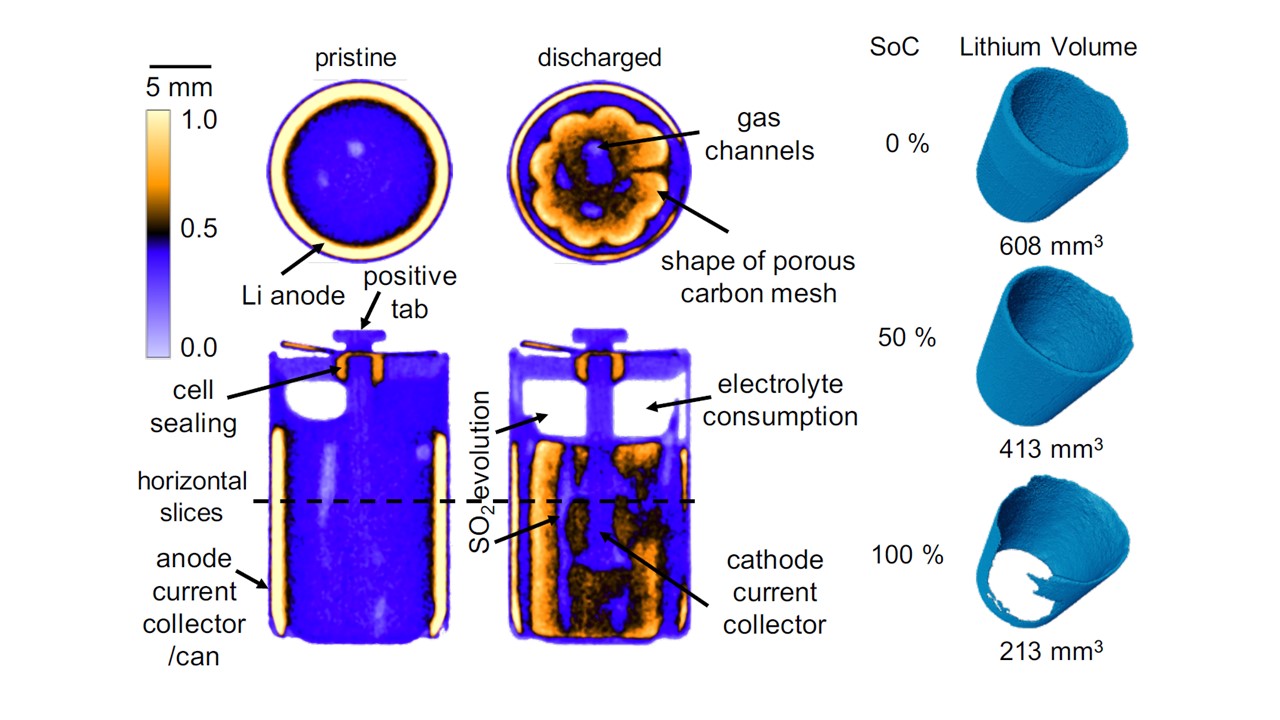
Figure 2: 3D reconstructed orthogonal slices visualising the intercalation in the cathode, electrolyte consumption in the cell head and lithium anode volumes of an LS 14250 Thionyl Chloride battery cell discharged with 8 mA.
One of Ralf’s experiments required a higher neutron flux instrument at the HZB Berlin, alongside X-ray experiments to look inside a battery with a slightly different structure. This study, published in Nature Communications, computationally ‘unravelled’ a commercial, spiral wound CR2 Li/MnO2 battery cell to investigate what was happening with each of the component parts. X-rays show structural changes such as electrode cracking and movement of active electrode material whereas neutrons enable the study of the electrochemistry such as electrolyte consumption and lithium diffusion. By using a novel virtual unrolling technique it was possible to analyse effects which are normally hidden by the spirally wound electrode structure.
Despite having a lower flux of neutrons, IMAT uses a pulsed source and offers the potential for studying the crystallography of operating batteries using neutron diffraction alongside Bragg edge imaging. The latter is an ongoing project with UCL, and this will enable future research to look at crystal structure changes of active electrode materials alongside the lithium diffusion.
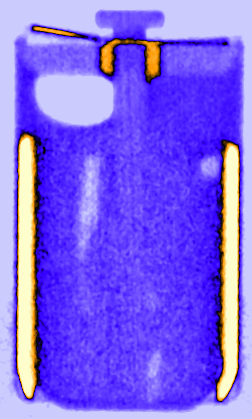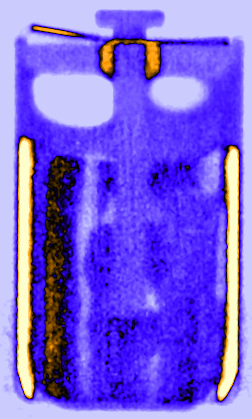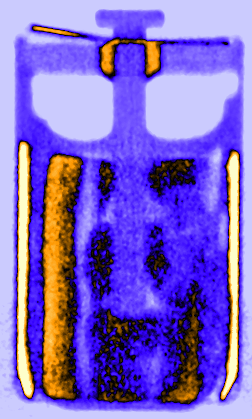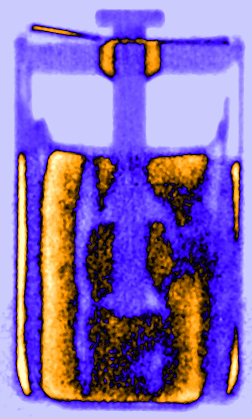
Figure 3: Time resolved vertical orthogonal slices of the LS 14250 cell discharged with 8 mA visualising the lithium diffusion from the lithium metal electrode in the liquid SOCl2 cathode.
“We are just at the beginning” says Ralf; “by developing new techniques on IMAT, we will be ready to make the most of the high-flux instruments at the ESS in terms of temporal and spatial resolution as well as continuing to leverage the unique benefits of ISIS and IMAT.”
Now having finished his PhD, Ralf has a post-doctoral research position with UCL and Diamond Light Source as part of the Faraday Institution Characterisation Project. “I really enjoyed the ability to be part of two organisations through the Facility Development Studentship” he says.
“I was able to take advantage of the knowledge and experience of my colleagues in both places, which made me more open to emerging analysis techniques and state-of-the art battery research alike. Being able to work at ISIS meant I could do experiments that are just not possible in the labs at a university.”
Further information:
Ralf’s two-part study, published in the Journal of The Electrochemical Society, can be found at DOI: 10.1149/1945-7111/abbbbc and DOI: 10.1149/1945-7111/abbfd9. His Nature Communications can be found online at DOI: 10.1038/s41467-019-13943-3

