Despite Mars regularly reaching temperatures well below freezing point, evidence is growing that liquid water once flowed on its surface and multiple sub-surface lakes of water remain. In a previous experiment, researchers from the University of Leeds, in collaboration with ISIS Neutron & Muon Source, explained how this may be possible. They are now applying their findings much closer to home.
The team found that magnesium perchlorate, Mg(ClO4)2, compresses the structure of water in a similar manner to large external pressure. This could prevent ice formation, with water remaining in a liquid state at sub-zero temperatures. One of their subsequent studies showed that TMAO, which is a naturally occurring osmolyte that protects proteins from denaturation, can partially restore this perturbation by restoring hydrogen bond networks.
Liquid water plays key structural and dynamical roles in biology, including acting as a 'universal solvent'. Just like flow on Mars, cellular water is affected by solutes.
In their most recent study, published in The Journal of Physical Chemistry, the researchers combined nuclear magnetic resonance (NMR), neutron diffraction on NIMROD and computational modelling. This provided a powerful tool to examine the solute-induced perturbations of both water structure and dynamics in solutions containing TMAO and Mg(ClO4)2 .
The two solutes cancelled out each other's perturbation; Mg(ClO4)2 weakened hydrogen bonds between the water molecules whereas TMAO strengthened them.
However, both solutes slowed the dynamics of water molecules present in the system. A particular hydrogen bond conformation, the bifurcated hydrogen, occurs as an intermediate step whilst a water molecule switches hydrogen bonding partners.
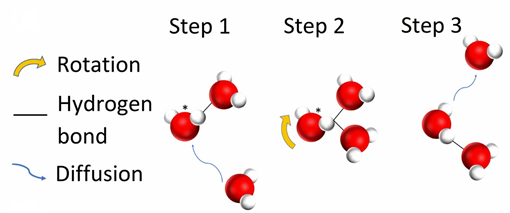
A water molecule switching hydrogen bonding partners, including the bifurcated hydrogen conformation occurring as an intermediate.
Their neutron diffraction and computational modelling revealed fewer hydrogen atoms in this conformation after TMAO and Mg(ClO4)2 addition. This signifies less frequent hydrogen bond switching events and therefore slower dynamics. NMR confirmed that the combined effect of the two solutes was additive.
Using a new quantification technique – plotting the data as a function of total solute concentration with an additional weighting factor – the researchers found that TMAO was 1.54 times more effective at perturbing water structure and dynamics than Mg(ClO4)2. This method could be applied to any complex aqueous system, making future studies on other solutes much easier.

Harrison Laurent, one of the researchers working on this project who is funded by an ISIS Facility Development Studentship, has recently returned to NIMROD to continue this research; “We are extending the study to solutions of larger biomacromolecules," He says; “By using the new DISSOLVE analysis software, created by ISIS facility scientist Dr Tristan Youngs, on NIMROD we can extend our previous studies to larger systems."
The next step is to find out whether the biological molecules can survive high physical pressures and understand how the addition of solutes might protect them against pressure denaturation. The combination of the new DISSOLVE structural refinement software and the NIMROD instrument enables a step change in the model systems which can be explored.
Photograph: Harrison Laurent on Nimrod in November 2020
Each of these steps forward in this area of research helps to build the scientific method needed to understand the natural limits to life on Earth, as well as intriguing clues to the possibility of life on Mars…
This project was funded by an EPSRC Fellowship grant (EP/P02288X/1) to Professor Lorna Dougan, School of Physics and Astronomy, University of Leeds and was a collaboration with Professor Michael Ries and Dr Daniel Baker, University of Leeds and Prof Alan Soper, ISIS facility.
The full paper can be found online at DOI: 10.1021/acs.jpcb.0c07780
