Supercapacitors are devices capable of absorbing and delivering large amounts of energy in the form of electric charge over short time-scales. Their excellent power handling characteristics make them an increasingly important class of energy storage devices that can store enough energy for a range of industrial applications such as automotive acceleration and braking or pulsed power applications in telecommunications. They achieve this capacitive increase by storing energy at a surface double-layer with an equivalent plate separation of 1 nanometre and have electrodes with surface areas of several hundred metres squared.
Most commercial supercapacitors use an organic liquid electrolyte, with the largest global manufacturers using acetonitrile. Although acetonitrile is mildly toxic, its relatively volatile, flammable nature restricts its use as an electrolyte for energy storage devices. This has led to a ban these types of supercapacitor to power buses in Shanghai when ambient temperatures exceed 35°C.
Dr Mojtaba Mirzaeian, from the University of the West of Scotland, and his team have begun developing an advanced range of supercapacitors with an aqueous electrolyte, rather than an organic electrolyte, to limit the problems of flammability and volatility. These advanced devices are more environmentally friendly and they can be manufactured in non-inert atmospheres – which reduces their overall cost. The main disadvantage is that these devices operate at much lower cell voltages and consequently have lower energy densities.
However, to compensate for their reduced energy density, Dr Mirzaeian's team developed carbon electrode materials with a very precisely controlled porosity and significantly larger working surface area than other devices.
The ideal situation would be one in which the aqueous electrolyte can access all of the pores within the carbon electrode. To increase the carbon/water interactions the group specially activated the carbons and loaded them with nitrogen heteroatoms.
To investigate the process taking place in these supercapacitors down to the nanoscale, Dr Mirzaeian and his team performed Contrast Matching Small Angle Neutron Scattering (CM-SANS) measurements on the LOQ instrument at ISIS.
The analysis of the CM-SANS data, below, showed that the activation process improves electrolyte accessibility to the carbon pores because the activation process leaves oxygen functional groups on the surface, which increases surface interactions with water molecules, increasing hydrophilic nature of the carbon.
The significantly lower scattering for the N-loaded sample also suggests that N-doping increases the accessibility of the electrolyte into the carbon pores. This is because nitrogen doping also increases the hydrophilic nature of the surface, further increasing carbon/water interactions.
Additionally, the O and N heteroatoms induce an element of pseudocapacitance, which contributes to a further increase in the performance of the carbon. These results show great benefits to the industrial application of supercapacitors. Although these carbons are more expensive, the cost difference will be offset by much cheaper manufacturing methods and safety improvements.
As Dr Mirzaeian says: “These measurements are to assist in the development of new supercapacitor devices that can store and release energy very quickly making electrified transport more affordable by extending battery pack lifetime." 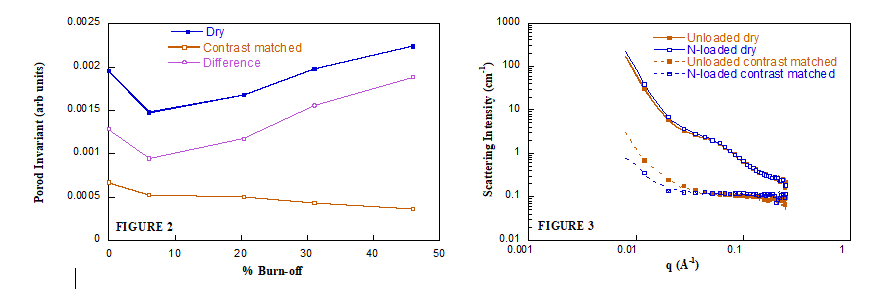
Figure 2: SANS from carbons activated to different degrees of burn-off. Figure 3: SANS from carbon loaded with nitrogen heteroatoms.
