Fuel cells convert hydrogen and oxygen into water, producing electricity with only water as a by-product. They are therefore of great interest for application in a greener economy. Conventional polymer electrolyte membrane fuel cells use catalysts to break hydrogen molecules into H+ ions, which then travel across a proton-conducting membrane. However, the catalysts used in these fuel cells are made of platinum-group metals, which are expensive and rare.
An alternative is a system with an anionic polymer exchange membrane (AEM) that relies on a polymer backbone decorated with ionic side groups to allow conduction of OH- ions. There are some of these already commercially available. To develop the next generation of improved membranes, it is crucial to understand and characterise the various coupled processes, and the dynamical interplay between them, as a function of operational parameters.
In this study, published in Nature Materials, a team of researchers used quasielastic neutron scattering across a range of timescales using the IRIS instrument at ISIS, the IN6-Sharp and IN16B instruments at the ILL, and HFBS at NIST to study a commercial anionic exchange membrane.
Through a comprehensive set of measurements using QENS, coupled with other characterisation techniques under operational conditions, they were able to shed light on the charge transport in an AEM fuel cell.
They were able to experimentally show that charge transport occurs via a combination of vehicular OH− diffusion and Grotthuss H+ exchange (suggested by previous simulations), with the two mechanisms balanced according to the operational conditions.
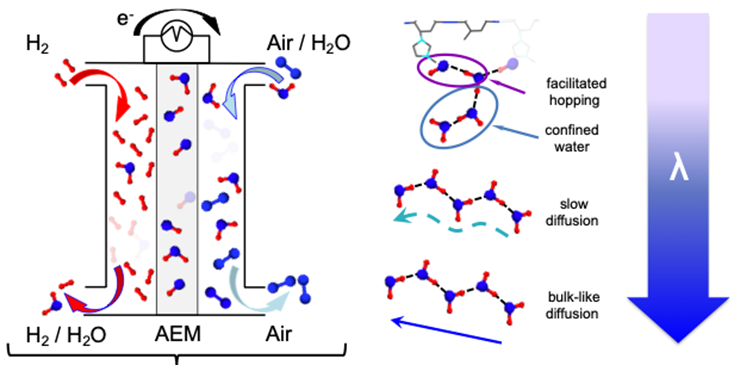
The study, using QENS to provide unique and detailed information about the coupling of local dynamics and vehicular diffusion, has proved essential to understand the conductivity mechanisms in greener solutions for polymer-based fuel cells.
The authors would like to dedicate this work to Professor Paul F. McMillan, who inspired this work, and who sadly passed away during the final stages of the manuscript revision.
Further information
The full paper can be found online at DOI: https://doi.org/10.1038/s41563-022-01197-2
