Extremophiles are organisms that are able to survive and thrive in extreme environments, enduring conditions such as high salinity, temperature, and pressure. These organisms are found in habitats inhospitable for humans all over Earth, from boiling and acidic volcanic areas to the deep depths of the Mariana Trench. Extremophiles that have adapted to high pressures are known as piezophiles. An example of their adaptation is the ability to accumulate organic molecules that ensure stability within the cells by maintaining the fine balance of interactions between biomolecules, such as proteins and DNA, and water.

The compound Trimethylamine N-oxide (TMAO), is one such example of these organic molecules
Previous studies show that organisms living under comparable pressures have similar levels of TMAO within their cells. This link between TMAO and pressure suggests a universal protective mechanism for life at high pressures. Previous studies have proposed that the TMAO molecule acts indirectly, meaning that it does not act on the biomolecule itself, but instead influences the water molecules surrounding the biomolecule.
We need to understand what happens to water under pressure and how pressure-adapted organisms combat these effects. If we can understand how these organisms survive at extreme pressure, we can apply these findings to our understanding of biomolecular stability in extreme conditions. Also, it can help to further our knowledge on the role of solutes, such as TMAO, in protein-protein assemblies and liquid-liquid phase separation, including those linked to pressure-induced neurological disorders.
While scientists have previously explored how TMAO perturbs water structure, and have studied the impact of high pressure on water structure, the potentially competing combination of TMAO and pressure on water structure is not clear. If this combination of conditions is understood, scientists can further expand their knowledge on how life survives at extreme pressures, and particularly the role of TMAO on water and biomolecules.
Using the NIMROD instrument at ISIS, the team from the University of Leeds, supported by ISIS scientists Dr Tom Headen, Dr Tristan Youngs and Prof. Alan Soper, investigated samples of either pure water or water with TMAO at two different pressures – an ambient pressure of 25 bar and a high pressure of 4 kbar (equivalent to 4000 times the atmospheric pressure on Earth at sea level). Using a combination of neutron diffraction, computational modelling and custom analysis developed by ISIS Facility Development student Dr Harrison Laurent, the researchers were able to build a 3D picture of the positions of the atoms within the sample, revealing the interactions between TMAO and water under pressure.
Harrison said-“Neutrons are really powerful for this study as they interact with the nucleus of an atom, unlike x-rays which interact with the electron clouds, and they interact with isotopes differently (including hydrogen vs deuterium). As our study focused on water structure, the unparalleled ability of neutrons to reveal subtle changes in the positioning of hydrogens between water molecules is of crucial importance for our understanding." This experiment also illustrates the penetrative ability of neutrons which had to pass through the walls of a thick metal pressure cell in order to reach the liquid sample. Nevertheless, the internal structure of the liquid could still be clearly determined.
Water has a tetrahedral structure which, when translated into a network, is sensitive to applied pressure. With increasing pressure, the tetrahedral network of water molecules collapses, and the structure becomes distorted, with the water-water hydrogen bonds becoming less stable. This can have an impact on biomolecules within organisms, as the hydrophilic and hydrophobic interactions between water molecules and biomolecules change.
The results show that TMAO acts to counteract the impact of high pressure, driving the water structure back to its ambient pressure-like conditions. TMAO does this by stabilising the water-water hydrogen bonding and resisting the destabilising effects of high external pressure. The molecule acts as an anchor point within the water network by providing a site to be able to form enhanced hydrogen bonds, which balances out the weaker water hydrogen bonding caused by increased pressure.
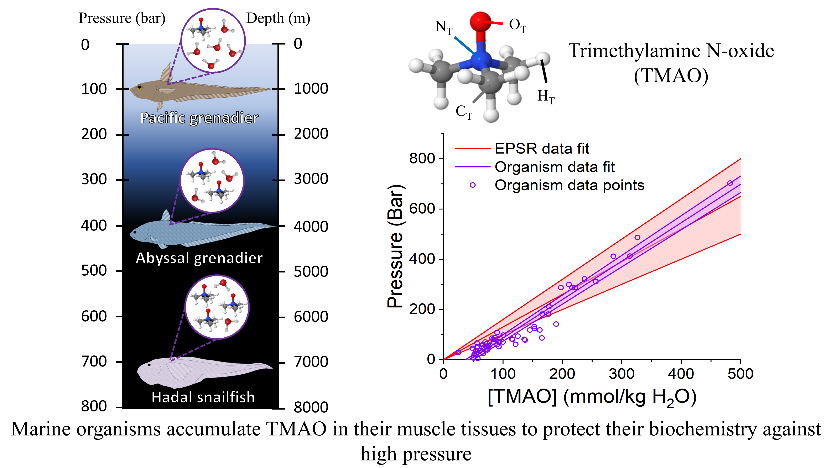 A chart showing the pressure-resisting ability of TMAO. This is calculated by considering the perturbation to bulk water-water hydrogen bonding by TMAO addition and pressure (red) through neutron scattering. Alongside is a figure showing the amount od TMAO in marine organisms relative to the pressure levels of where they inhabit.
A chart showing the pressure-resisting ability of TMAO. This is calculated by considering the perturbation to bulk water-water hydrogen bonding by TMAO addition and pressure (red) through neutron scattering. Alongside is a figure showing the amount od TMAO in marine organisms relative to the pressure levels of where they inhabit.
Professor. Dougan said-“Previous studies have observed a scaling relationship between the amount of pressure-protecting molecule TMAO found in the muscle of organisms and the increasing depths (and pressures) they inhabit. Remarkably, we find that considering only the water and the impact of pressure and TMAO, we can recapture the scaling relationship between TMAO and pressure. Our study bridges single molecule perturbations to water with the whole organism studies of fish living at high pressures."
The team at the University of Leeds have created an animated video on their research and findings:
Supported by ESPRC and Royal Academy of Engineering, the team have also developed Maker kits which allows the public to explore this research. See Extreme Water Maker Kits for more information.
Written by Rupo Mapanga and Caitlin Convey.
The full paper can be found online at DOI: 10.1038/s42004-022-00726-z
