Enveloped viruses are a class of pathogens responsible for numerous epidemics in recent years. Ebola, influenza, human immunodeficiency virus (HIV), and perhaps most notably of late, coronaviruses are enveloped viruses. Their significant impact on human lives is undeniable and to avoid further epidemics, there is a need to develop methods of defense against such viruses. This study investigated the potential of the antimicrobial peptide LL-37 produced by the human immune system to thwart these viruses, by learning more about its mechanism of action.
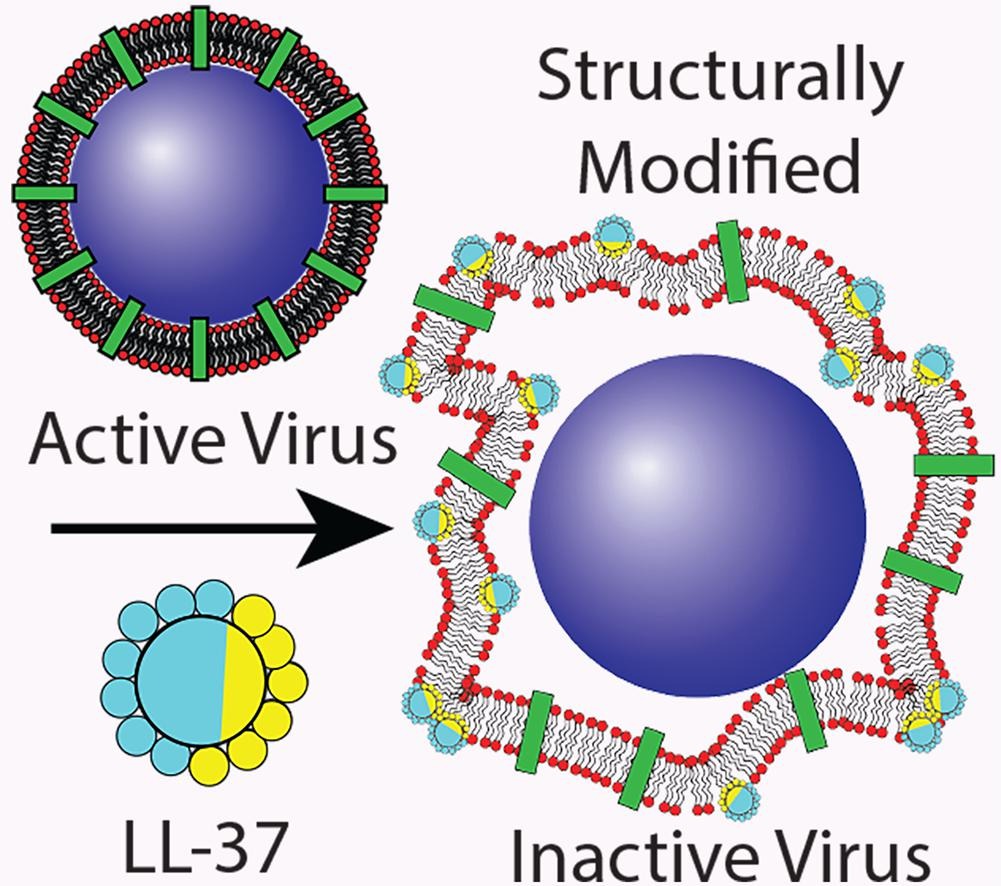 At the core of enveloped viruses, genetic material is surrounded by a protein shell called a nucleocapsid. The nucleocapsid is surrounded by a lipid bilayer envelope in which host cell recognition proteins are embedded. These structural features are common to all enveloped viruses. The envelope has been identified as a potential weak spot in the virus’ structure and can be targeted by nanomedicines. Antimicrobial peptides (AMP) are naturally occurring compounds known to inactivate microbes by impairing the barrier ability of the lipid bilayer. The human cathelicidin peptide LL-37 is an antimicrobial peptide produced by the human innate immune system and a promising antiviral therapeutic with the potential to limit the activity of several viruses, including influenza A, HIV, dengue, and others. However, the mechanism of this interaction is mostly unknown.
At the core of enveloped viruses, genetic material is surrounded by a protein shell called a nucleocapsid. The nucleocapsid is surrounded by a lipid bilayer envelope in which host cell recognition proteins are embedded. These structural features are common to all enveloped viruses. The envelope has been identified as a potential weak spot in the virus’ structure and can be targeted by nanomedicines. Antimicrobial peptides (AMP) are naturally occurring compounds known to inactivate microbes by impairing the barrier ability of the lipid bilayer. The human cathelicidin peptide LL-37 is an antimicrobial peptide produced by the human innate immune system and a promising antiviral therapeutic with the potential to limit the activity of several viruses, including influenza A, HIV, dengue, and others. However, the mechanism of this interaction is mostly unknown.
This study examined the impact of LL-37 on the enveloped bacteriophage Phi6, an RNA virus. Phi6 is used as a surrogate virus for viral pathogens like SARS-CoV-2 and Ebola. Information on the nanostructure of the virus was gained via small-angle X-ray and neutron scattering (SAXS and SANS) complemented with cryogenic transmission electron microscopy. Multi-angle dynamic light scattering (DLS) was used to observe changes in virus size. Biological infectivity assays allowed study of the virus’ biological activity.
SANS measurements performed at ZOOM and SAXS experiments revealed that LL-37 actively integrates into the lipid bilayer of Phi6’s envelope. Depending on the LL-37 concentration, this integration resulted in structural modifications of the virus envelope, and a significant increase in the particle size as shown by DLS. Biological assays confirmed the activity loss at these concentrations, suggesting that the loss in biological function results from the LL-37 triggered structural modifications in Phi6’s envelope. Overall, these data suggest that the structural changes of the lipid envelope result in the loss of infectivity by separating the host recognition function in the lipid envelope from the genetic information in the nucleocapsid.
In conclusion, LL-37 could inactivate more than 99.99% of Phi6 particles at high enough concentrations and can thus be classified as virucidal. This infectivity loss of Phi6 is triggered by the integration of LL-37 into the Phi6 envelope structure, leading to modifications of the packaging of the envelope molecules. This eventually results in the separation of the envelope from the nucleocapsid and, hence, the loss of the host recognition function. These findings improve the understanding of the structure-activity relationship of enveloped viruses and the antiviral mechanism of the peptide LL-37. The non-specific nature of the mechanism suggests that LL-37 may be used as a template for future development of wide-spectrum drugs with little risk for pathogens to develop resistance against the drug.
