In England, we use ~14 billion litres of water each day, all of which undergoes industrial water treatment processes before it can be supplied to homes and businesses before use. Normal surface water (the kind that falls as rain and enters our rivers and lakes) can contain large amounts of NOM (natural organic matter), of which, humic acid is a major component. Humic acid (HA) gives natural water its brownish colour, indicating to most of us a lack of cleanliness. It is made up of a large variety of organic macromolecules with carboxylic and phenolic groups, resulting in a negative surface charge.
The complexation of HA by polycations is a step in the coagulation stage of water treatment, forming rather neutral precipitates in water. This paper studied the interaction between oppositely charged humic acid (HA) and the polycation polydiallyldimethylammonium chloride (PDADMAC), which is widely used in conventional water treatment. They specifically focused on the effects of Ca2+ as it is inevitably present in water under real condition, but little is known of its impact on the process.
Using a combination of light scattering and Small Angle Neutron Scattering (SANS), they studied phase behaviour and structure of the solution. Their findings suggest that the presence of Ca2+ improves the effectiveness of the water treatment process. They saw it increase the neutralisation power, reducing the amount of PDADMAC required to precipitate HA during the water treatment process. HA flocs also become more densely packed in the presence of Ca2+, with enhanced structural stability that mean they are less likely to be redispersed during the agitation and transportation processes that occur during water treatment. Floc formation and agglomeration are observed earlier during the mixing of HA and PDADMAC when Ca2+ is present and UV-vis spectrometry showed increasing the concentration of Ca2+ can cause higher HA removal.
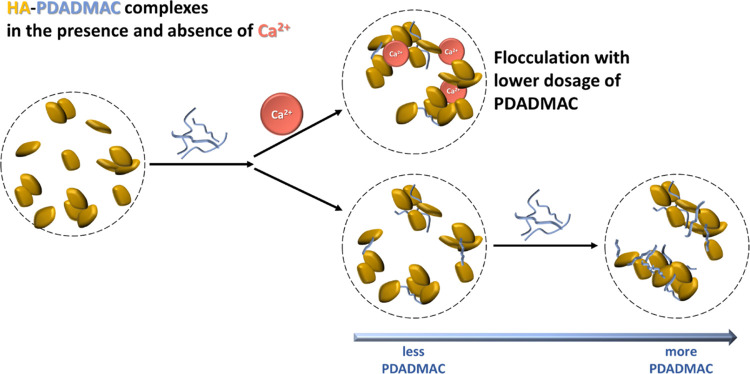
A schematic drawing of the complexation between cationic PDADMAC and anionic HA in the presence and the absence of Ca2+.
This study was also the first time the colloidal structures of HA-PDADMAC complexes had been observed, using light and neutron scattering. Here the effect of Ca2+ was small but clear, showing to promote the PDADMAC compaction of aggregates formed with HA. Further SANS probing with Mg2+ and Na+ ions, performed on Sans2d, also showed Ca2+ has by far the strongest effect on the complexes formed, due to its strong interaction with the carboxylic groups of HA.
This study shows that the presences of Ca2+ during the precipitation process leads to a more effective removal of HA. This is of high relevance to the practical use of polycations in NOM removal in the water industry and could offer insight to optimise the water treatment process in industrial fields. The researchers also hope that the characterisation of the HA-PDADMAC complexes can be used as a basis the development of polycations from natural resources to contribute to the development of more environmentally friendly solutions.
Read the full paper: https://pubs.acs.org/doi/10.1021/acs.langmuir.3c03029?goto=supporting-info
