Some animals, particularly ruminants and termites can digest cellulose with the aid of symbiotic micro-organisms that live in their guts, however we cannot; and the reasons behind the persistent insolubility of cellulose is under investigation with hope of future biofuel applications.
The SANDALS instrument at ISIS was used by Dr Sylvia McLain, University of Oxford, who worked with former undergraduate of the University of Tennessee, Brad O’Dell to look at the interactions of water molecules with cellobiose, a basic building block of cellulose, in hope of uncovering the reason why cellulose is not water-soluble.
It is estimated that the solubility of cellulose is affected by which two carbons are linked by the glycosidic bond. If the physics behind cellobiose in solution can be understood, there is the potential for mechanisms to convert cellulose into ethanol, which can be used as a biofuel.
“ISIS has the only instrument capable of doing this work, it’s the best in the world” says Sylvia. “One of the things that ISIS does very well is hydrogen studies. SANDALS has a large angular range, and it’s been used for years to do hydrogen-deuterium substitution, which not a lot of other establishments do.”
Neutron scattering tells a lot about hydrogen positions and the structure of water around the cellobiose molecule, which is important in understanding the interactions between water and cellobiose.
The investigation revealed that the cellobiose molecule competes with water to form an inter-residue hydrogen bond between the two sugar molecules found in cellobiose.
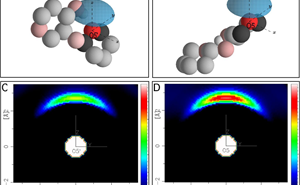
The distribution of water molecules (red and dark grey) about the oxygen (pink) in cellobiose.
View full-size image
“There is direct evidence of water competing with cellobiose to bind to the non-reducing 5’ oxygen” reiterated Sylvia. This intermolecular bonding would exclude water from bonding to the molecule and may explain its insolubility in water, particularly when it comes to longer chains of cellobiose molecules. This has implications for biofuel production from plant matter, where in plants the natural recalcitrance to water has to be overcome to break down cellulose for use as a feedstock for biofuels.
Additionally, there was other value that came out of this investigation. Sylvia and her team combined a variety of experimental techniques – neutron diffraction with isotopic substitution, NMR spectroscopy and computation – which allowed for a complete assessment of the molecule in question.
“The kind of research you can get from applying several techniques is greater than the sum of its parts. Learning how to combine NMR with the computer model to combine again with the neutron data is a big step forward” Sylvia recapped.
This in turn can help understand the principles behind why all these things happen in life. If a map of hydration around different, complex biological molecules can be constructed, it may help in understanding phenomena such as why proteins fold, another area of Sylvia’s research.
Future studies which hold the potential to return to ISIS include measuring longer and longer chains of cellobiose and other cellulose building block molecules, to see if there is still the same hydrogen bonding motif.
Emily Mobley
Sylvia McLain et al.
Research date: October 2012
Further Information
Further reading:
O'Dell WB, Baker DC, McLain SE (2012) Structural Evidence for Inter-Residue Hydrogen Bonding Observed for Cellobiose in Aqueous Solution. PLoS ONE 7(10): e45311.
DOI: 10.1371/journal.pone.0045311
McLain group website: http://www2.bioch.ox.ac.uk/mclaingroup/index.html
